General Description
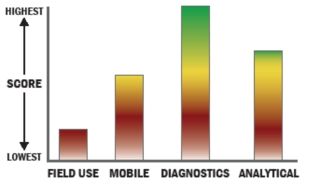
The EncompassMDx™ is a fully-automated, modular, molecular analytical system designed to perform a broad range of molecular tests on a wide variety of sample types. The instrument can run up to 24 samples through a fully integrated molecular assay with no user intervention, automatically manipulating reagents internally on the Rheonix CARD® cartridge. The system is well suited for in vitro diagnostic (IVD) assays, industrial and environmental applications, and user-defined protocols for life science research applications. Key features and benefits of the EncompassMDx system include: - Scalable sample throughput to enable work in small clinics or large labs
- A sample-to-result platform that consolidates multiple pieces of equipment
- Simple interface requiring only minimal training for all skill levels
- Ability to sample liquid volume between 5 µl to 5 ml and tissue mass up to 20 mg
- Highly multiplexed detection to screen many targets in one test
- Configurable design for operating many purification and detection methodologies on a single instrument
Technical Description
The EncompassMDx™ fully-automated molecular platform can detect the nucleic acid sequences from a wide variety of samples. Once a sample is applied to the cartridge, all assay steps are automatically performed. Within the purification portion of the CARD cartridge, cells are lysed and DNA is extracted and purified. The purified DNA sample is directed into PCR reaction chambers where targets are amplified. Amplified products are denatured and flow into the microarray portion of the CARD cartridge. Hybridization is detected using a streptavidin-conjugated horseradish peroxidase (HRP)-mediated color development system. The EncompassMDx™ detects the precipitated reaction products and reports a result.
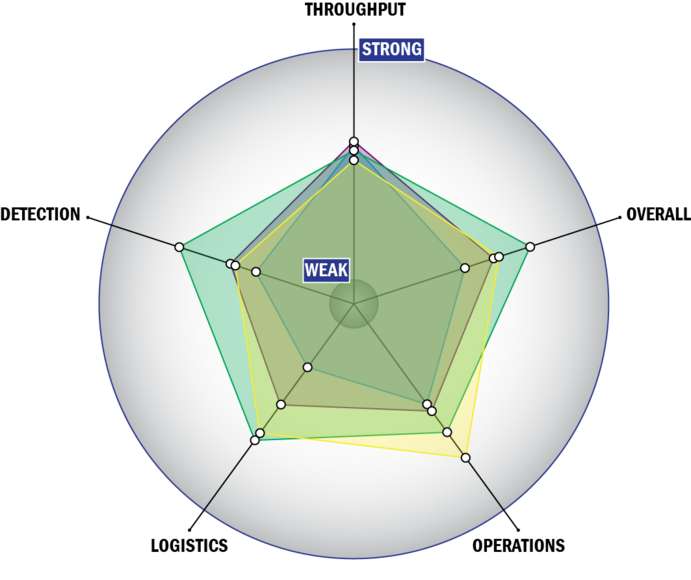
Evaluation Criteria
THROUGHPUT:
- Between 60 minutes and 8 hours for detection
- Multiple samples, multiple tests/sample per run
- Less than 32 samples every 2 hours
- The system or device is currently fully automated
- Device or system is designed for a single use
- 2 solutions, buffer, eluents, and/or reagents
- 4 components
- Less than 5 minutes is required for set-up
- Automatic detection
LOGISTICS:
- Very brief (minutes-hours) training and minimal technical skills
- Approximately the size of a home dishwasher
- More than 50 kg
- This system is not capable of transmitting data
- System or device has 110V electrical requirement
OPERATIONS:
- Can be used from 25°C to 37°C
- Components must be stored at 4°C
- 3-5 years expected life
- Results can be viewed in real-time
- The system could be adapted to a fully autonomous system with significant effort
- The system software is open and available for modification
- The system hardware is closed and not available for modification
DETECTION:
- Efforts are underway to achieve 510K clearance
- Efforts are underway to achieve FDA approval
- Less than 100 µL
- 1-100 CFU per mL
- Fully automated spore lysis




 Top Tier
Top Tier Second Tier
Second Tier Third Tier
Third Tier Fourth Tier
Fourth Tier Bottom Tier
Bottom Tier