General Description
The FilmArray System is a user friendly automated pathogen identification system. The dual use platform with integrated sample preparation is capable of analyzing clinical and environmental samples automatically testing for multiple pathogens simultaneously from a raw sample. The lightweight, small footprint instrument processes a sample, analyzes the data and reports results in one hour. Consumable pouches are freeze dried and room temperature stable. ITI’s current test panels are for respiratory disease and biothreat with its development pipeline to include, gastrointestinal disease, sepsis, and sexually transmitted disease panels. The system is intended for clinical diagnostic use and environmental testing. It is simple to use requiring little training and net-work capable with connectivity to the national health information network and composite health care system available.
Technical Description
The FilmArray integrates sample preparation into a highly multiplexed Polymerase Chain Reaction (PCR) system developed for the point-of-care diagnostic market. The single sample instrument uses a consumable pouch that integrates sample preparation and nested multiplex PCR. Integrated sample preparation provides ease-of-use while the highly multiplexed PCR provides both the sensitivity of PCR and the ability to test for up to 30 different organisms simultaneously.
Notes:
Selected as Next Generation Diagnostics System (NGDS) prototypes.
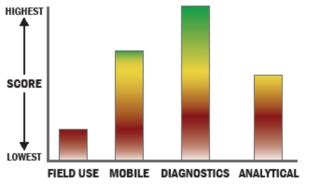
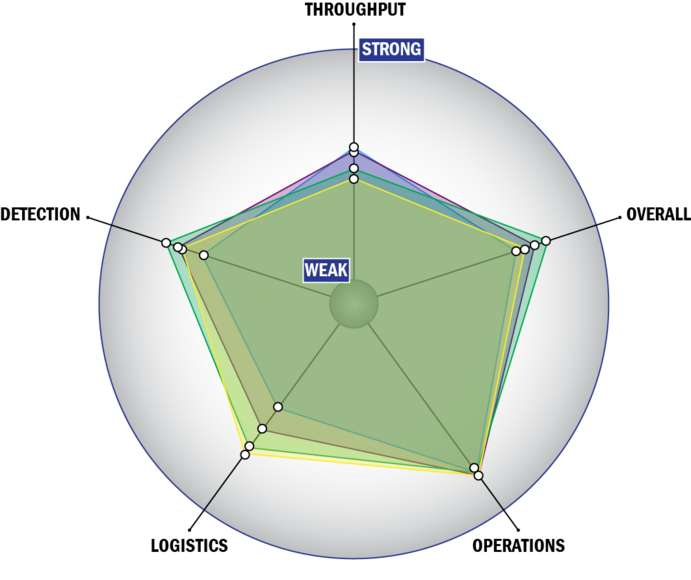
Evaluation Criteria
THROUGHPUT:
- Between 30 and 60 minutes for detection
- 1 sample, >10 tests/sample per run
- Less than 32 samples every 2 hours
- The system or device is currently fully automated
- Device or system is intended for multiple detection assays
- 2 solutions, buffer, eluents, and/or reagents
- 3 components
- Less than 5 minutes is required for set-up
- 3-5 steps are required for detection
LOGISTICS:
- Very brief (minutes-hours) training and minimal technical skills
- Approximately the size of a toaster
- Between 5 and 25 kg
- Wireless and wired connections are available
- System or device has 110V electrical requirement
OPERATIONS:
- Can be used from 4°C to 41°C
- Components must be stored at room temperature (27°C)
- Performance is not influenced by relative humidity
- Between 6 months and 1 year shelf life
- Greater than 10 years expected life
- Results cannot be viewed in real-time
- The system could be adapted to a fully autonomous system with some effort
- The system software is closed and not available for modification
- The system hardware is open and available for modification
DETECTION:
- System currently has 510k clearance
- System currently has FDA approval
- Less than 250 µL
- Excellent specificity. System has occasional false alarms under certain conditions (<2%)
- 100-1,000 CFU per mL
- 1,000-10,000 PFU per mL
- Fully automated spore lysis




 Top Tier
Top Tier Second Tier
Second Tier Third Tier
Third Tier Fourth Tier
Fourth Tier Bottom Tier
Bottom Tier