General Description
The Fluorescent Assay Sandwich Handheld (FLASH) Reader is a second generation portable fluorometer with epifluorescence optics designed to rapidly and sensitively assess fluorescence emanating from magnetic bead-based sandwich assays. In particular, the FLASH Reader is customized to read OpTech's DNA Aptamer-based magnetic bead sandwich assays for a variety of foodborne pathogens, arboviruses, rickettsia and Leishmania parasites.
Technical Description
The basic technology centers on epifluorescence using a 630 nm red laser and PMT to read DNA aptamer-based magnetic bead sandwich or FRET assays.
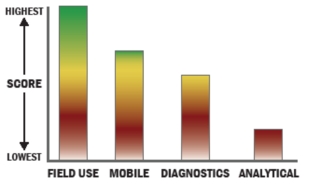
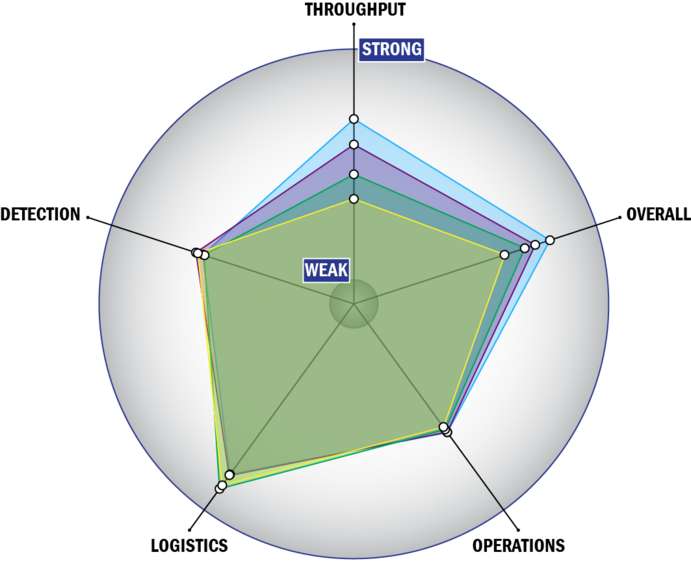
Evaluation Criteria
THROUGHPUT:
- Between 30 and 60 minutes for detection
- 1 sample, single test/sample per run
- Less than 32 samples every 2 hours
- The system or device is currently semi-automated
- Device or system is intended for multiple detection assays
- 0-1 solutions, buffer, eluents, and/or reagents
- 2 components
- Less than 5 minutes is required for set-up
- 1-2 steps are required for detection
LOGISTICS:
- Very brief (minutes-hours) training and minimal technical skills
- Approximately the size of a toaster
- Between 1 and 5 kg
- Wired connections are available
- System or device uses batteries
- 4-8 hours battery life
OPERATIONS:
- Can be used from 4°C to 37°C
- Components must be stored at room temperature (27°C)
- Device or system has peak performance at normal relative humidity conditions
- Between 1 to 3 years shelf life
- Greater than 10 years expected life
- Results can be viewed in real-time
- The system could be adapted to a fully autonomous system with significant effort
- The system software is open and available for modification
- The system hardware is open and available for modification
DETECTION:
- Possible the system could receive 510K clearance, no current efforts at this time
- Possible the system could receive FDA approval, no current efforts at this time
- Greater than 250 µL
- Good specificity. Consistently low level of false alarms (2-5%)
- 1-100 CFU per mL
- 100-1,000 PFU per mL
- 1-10 ng per mL
- Spore lysis not necessary for detection by system
- 3x10-5 - 1x10-4 mg/m3
- 1 ppm – 100 ppm




 Top Tier
Top Tier Second Tier
Second Tier Third Tier
Third Tier Fourth Tier
Fourth Tier Bottom Tier
Bottom Tier