General Description
MedMira’s patented rapid flow-through technology platform is the basis of the Company’s rapid tests Key features of the rapid tests: - A 3-minute procedure that produces instant results
- Capabilities to test whole blood, serum or plasma specimens as well as others
- Multiplexing capabilities; multiple results on a single cartridge using a single specimen
- Up to a 24 month shelf-life at 2-30° C
- A compact, single-use, 0.7 oz. package
- No refrigeration required
- A built-in procedural and reagent control line
- A standardized procedure across all products
The Company’s current product line includes single rapid tests for HIV, Syphilis and H. Pylori, and multiplex tests for HIV, Hepatitis B and C, and Syphilis in various combinationsThe Company’s quality management system is ISO9001:2008 and ISO13485:2003. MedMira’s rapid tests and technology platform have been evaluated and approved by the world’s leading regulatory agencies.
Technical Description
MedMira’s patented rapid flow-through technology is a highly versatile product engine, enabling our team to quickly move new rapid testing applications through the discovery, design and development, and clinical phases to full commercialization.
The technology facilitates the formation of highly specific antigen-antibody reactions allowing disease-specific biomarkers in human whole blood, serum or plasma to be captured and visualized on a unique membrane. The simple test procedure involves adding the specimen to the device and allowing it to flow through the membrane. If the specimen contains the target antibodies or antigens, they are captured on the test membrane and can be visually interpreted immediately after the addition of a detection reagent. Our technology platform is unique in its ability to detect multiple biomarkers specific to several diseases using a single cartridge. Precision pipetting, sample manipulation, specialized equipment and training are not required to perform any of MedMira’s rapid tests, making it an invaluable diagnostic resource in a broad range of settings.
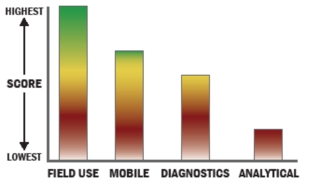
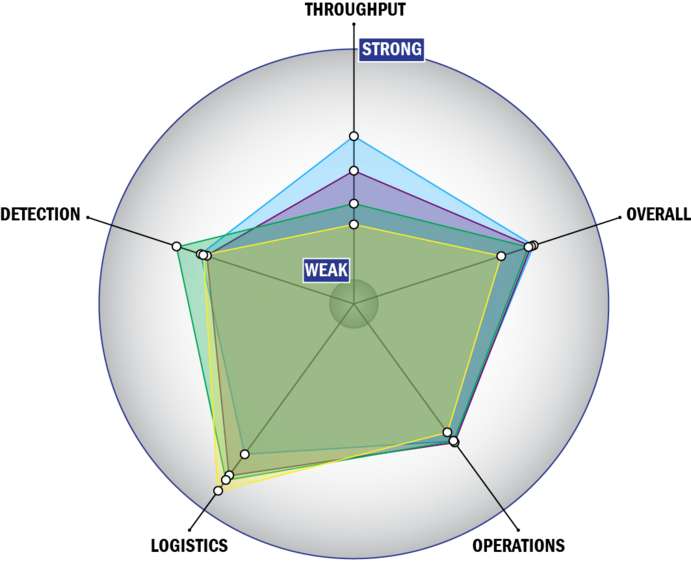
Evaluation Criteria
THROUGHPUT:
- Between 2 and 15 minutes for detection
- 1 sample, single test/sample per run
- 95-32 samples every 2 hours
- The system or approach is not amenable to full or semi-automation
- Device or system is designed for a single use
- 0-1 solutions, buffer, eluents, and/or reagents
- 0 components
- No set-up of the system is required
- 3-5 steps are required for detection
LOGISTICS:
- Very brief (minutes-hours) training and minimal technical skills
- Approximately the size of a soda can
- Less than 1 kg
- This system is not capable of transmitting data
- There is no electrical requirement
OPERATIONS:
- Can be used from 4°C to 37°C
- Components must be stored at room temperature (27°C)
- Performance is not influenced by relative humidity
- Between 1 to 3 years shelf life
- Results can be viewed in real-time
- The system is not capable of autonomy
- The system does not employ any software
DETECTION:
- Possible the system could receive 510K clearance, no current efforts at this time
- System currently has FDA approval
- Less than 50 µL
- Superior specificity. System has a false alarm rate approaching zero (~0%)
- 1,000-10,000 CFU per mL
- 1-10 ng per mL
- Spore lysis not necessary for detection by system
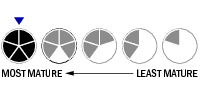



 Top Tier
Top Tier Second Tier
Second Tier Third Tier
Third Tier Fourth Tier
Fourth Tier Bottom Tier
Bottom Tier