General Description
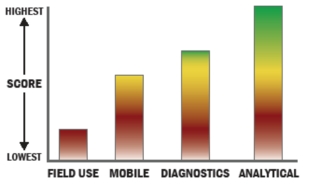
The SECTOR® PR2 instrument product line uses MSD’s MULTI-ARRAY® electrochemiluminescence (ECL) technology to carry out highly sensitive, multiplexed immunoassays for biothreat agents. The PR2 instruments work with MSD’s MULTI-ARRAY® 96-well assay plates. Each well of the plate has an array of up to 25 different antibodies to enable multiple targets (including bacteria, viruses, toxins and internal process controls) to be measured simultaneously. To provide stability and ease of use, the plates are sealed and contain all the required antibody reagents in dry format. The PR2 product line has been used to test a wide variety of sample types including many clinical sample types, dry filter unit extracts, aerosol samples, food and beverage samples, water, and soil samples. MSD’s MULTI-ARRAY biodefense assays have undergone a range of sensitivity, near neighbor, interferent, and suspicious powder testing. The PR2 Model 1500 instrument is designed for OEM use in aerosol, water, or other sampling applications where autonomous sample analysis is desired. The Model 1500 includes a sample reservoir that accepts a 1mL liquid sample from the host system. The Model 1500 can then be commanded via the instrument’s Ethernet interface to analyze the sample and provide results back to the host system in XML format. Time to result can be as short as 15 minutes. The instrument can accept a new liquid sample every five minutes and process multiple samples in parallel.
Technical Description
MSD’s PR2 and Cartridge Reader instruments employ MSD’s MULTI-ARRAY® technology that combines electrochemiluminescence (ECL) detection and array-based multiplexed measurements. ECL immunoassays enable highly sensitive measurement of samples for the presence of bacteria, viruses, and toxins. The measurements are performed on arrays printed on carbon ink electrodes that are incorporated into multi-well assay plates and cartridges. MULTI-ARRAY technology consistently has been demonstrated to provide high performance measurement capability in a wide range of matrices and in the presence of many interferents. While MULTI-ARRAY technology is primarily used for immunoassays, the technology can also be used for multiplexed nucleic acid measurements.
Notes:
This system has been selected for future fielding by the U.S. DoD.
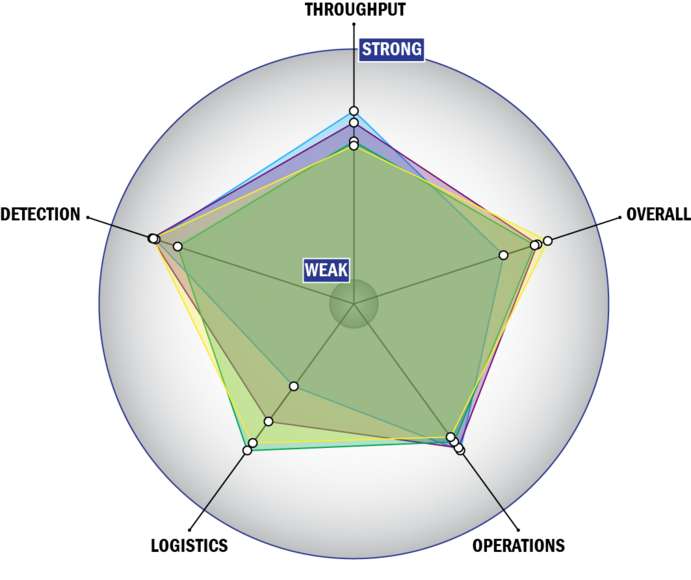
Evaluation Criteria
THROUGHPUT:
- Between 15 and 30 minutes for detection
- Multiple samples, multiple tests/sample per run
- Less than 32 samples every 2 hours
- The system or device is currently fully automated
- Device or system is intended for multiple detection assays
- 0-1 solutions, buffer, eluents, and/or reagents
- 1 component
- Less than 5 minutes is required for set-up
- Automatic detection
LOGISTICS:
- A day of training and technical skills are required
- Approximately the size of a carry-on luggage suitcase
- Between 25 and 50 kg
- Wired connections are available
- System or device has 110V electrical requirement
OPERATIONS:
- Can be used from 4°C to 41°C
- Components must be stored at room temperature (27°C)
- Performance is not influenced by relative humidity
- Between 1 to 3 years shelf life
- 5-10 years expected life
- Results cannot be viewed in real-time
- The system or device is currently fully autonomous
- The system software is closed and not available for modification
- The system hardware is closed and not available for modification
DETECTION:
- Possible the system could receive 510K clearance, no current efforts at this time
- Possible the system could receive FDA approval, no current efforts at this time
- Greater than 250 µL
- Superior specificity. System has a false alarm rate approaching zero (~0%)
- 100-1,000 CFU per mL
- 100-1,000 PFU per mL
- Less than 1 ng per mL
- Spore lysis not necessary for detection by system




 Top Tier
Top Tier Second Tier
Second Tier Third Tier
Third Tier Fourth Tier
Fourth Tier Bottom Tier
Bottom Tier