General Description
The 451P state-of-the-art ion chamber survey meter is a handheld battery operated unit designed for use in both rugged and normal environments. The 451P is a pressurized ion chamber for μR resolution. The 451P has auto-ranging and measure radiation rate and accumulated dose from various radiation sources (x-ray and gamma). The ion chamber detector allows for a fast response time to radiation from leakage, scatter beams, and pinholes. Additionally, the low-noise chamber bias supply provides for fast background-settling time.
Technical Description
Pressurized ion chamber technology.
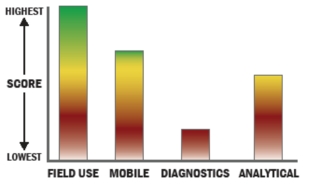
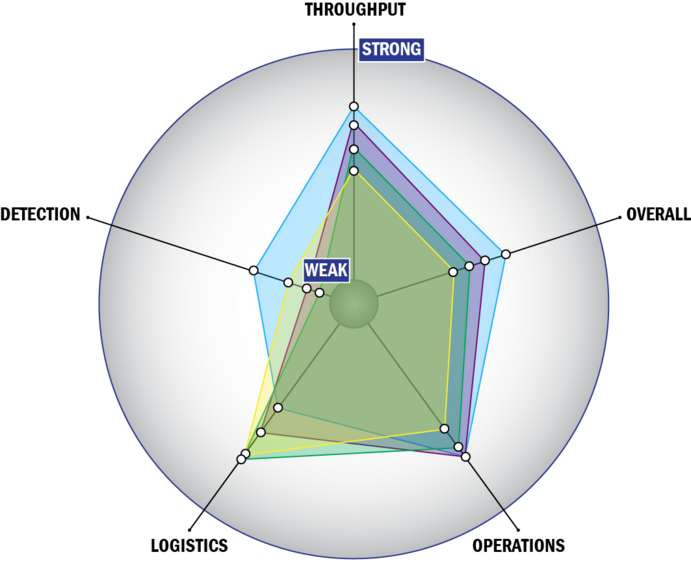
Evaluation Criteria
THROUGHPUT:
- 2 minutes or less for detection
- 1 sample, single test/sample per run
- System is continuous and provides real time analysis with no defined tests/samples
- The system or approach is not amenable to full or semi-automation
- Device or system is intended for multiple detection assays
- 0-1 solutions, buffer, eluents, and/or reagents
- 1 component
- Less than 5 minutes is required for set-up
- 1-2 steps are required for detection
LOGISTICS:
- Very brief (minutes-hours) training and minimal technical skills
- Approximately the size of a toaster
- Between 1 and 5 kg
- System or device uses batteries
- Is commercially available
OPERATIONS:
- Can be used from < -21°C to > 42°C (All temperatures)
- This system does not require consumable components
- Performance is not influenced by relative humidity
- Greater than 3 years shelf life
- 5-10 years expected life
- Results can be viewed in real-time
- The system is not capable of autonomy
- The system does not employ any software
- The system is single use or this question does not apply to this device
DETECTION:
- Not possible for the system to achieve 510K clearance
- Not possible for the system to achieve FDA approval
- This system does not test liquids and this question does not apply
- Excellent specificity. System has occasional false alarms under certain conditions (<2%)
- Displays only total dose and dose rate
- Down to background level radiation detection (i.e., gamma 1 uR/hr)
- This system does not measure count rate
- System is used for surveying




 Top Tier
Top Tier Second Tier
Second Tier Third Tier
Third Tier Fourth Tier
Fourth Tier Bottom Tier
Bottom Tier