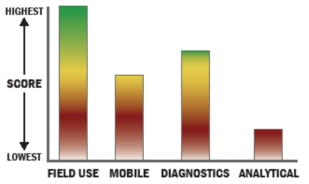
General Description
Total ATP tests have been developed (3M Clean Trace) in recent years, but they measure total biomass and do not differentiate ATP derived from bacteria. We have taken the proven total ATP methodology; improved sensitivity, eliminated false positives from non-bacteria cells, and eliminated false negatives from quenching agents often present in these samples. Thus we have a rapid, point-of-care bacteria detection system that only detects live bacteria. The test system is portable and can be taken into the field for monitoring of bacteria and easily used by non-laboratory personnel. Test time is under one minute. Detection limits go down to 100 CFU. The product is commercial and currently is used for bacteria detection in healthcare, food and water applications.
Technical Description
This kit detects the presence of living bacterial cells by measuring the amount of ATP in the sample. ATP is a cellular metabolite present in all living cells; the amount of ATP in a sample is proportional to the number of living cells. The test can distinguish bacteria from human cells through the use of sample preparation methods that release ATP selectively from microbial cells. The system has two main components: a microluminometer to read ATP-induced luminescence and a "Filtravette" (a filter device and cuvette combined) to concentrate cells from a sample solution and remove chemical contaminants. Samples and solutions are processed through the filtravette using an empty syringe. After the sample is processed, a solution is applied to remove non-microbial sources of ATP. Then a microbial lysis solution is added to the filtravette followed by a luciferase solution that produces light in the presence of ATP. After pipette mixing, the filtravette is placed in the reader and the intensity of emitted light (luminescence) is recorded.
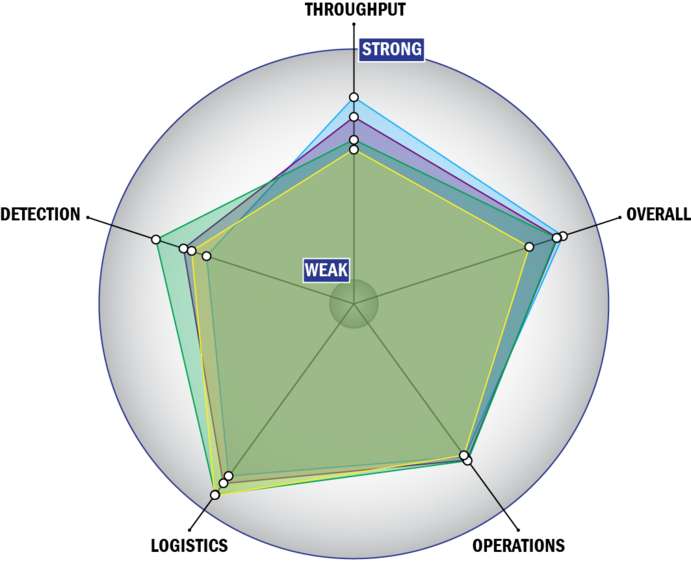
Evaluation Criteria
THROUGHPUT:
- 2 minutes or less for detection
- 1 sample, single test/sample per run
- 349-96 samples every 2 hours
- The system could easily be adapted into a fully automated system
- Device or system is intended for multiple detection assays
- 2 solutions, buffer, eluents, and/or reagents
- 1 component
- No set-up of the system is required
- 3-5 steps are required for detection
LOGISTICS:
- Very brief (minutes-hours) training and minimal technical skills
- Approximately the size of a soda can
- Less than 1 kg
- Wired connections are available
- System or device uses batteries
- 4-8 hours battery life
OPERATIONS:
- Can be used from 4°C to 41°C
- Components must be stored at room temperature (27°C)
- Performance is not influenced by relative humidity
- Between 6 months and 1 year shelf life
- 3-5 years expected life
- Results can be viewed in real-time
- The system could be adapted to a fully autonomous system with some effort
- The system software is open and available for modification
- The system hardware is open and available for modification
DETECTION:
- System currently has 510k clearance
- System currently has FDA approval
- Less than 50 µL
- Excellent specificity. System has occasional false alarms under certain conditions (<2%)
- 1-100 CFU per mL
- Semi-automated spore lysis




 Top Tier
Top Tier Second Tier
Second Tier Third Tier
Third Tier Fourth Tier
Fourth Tier Bottom Tier
Bottom Tier