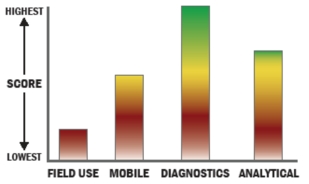
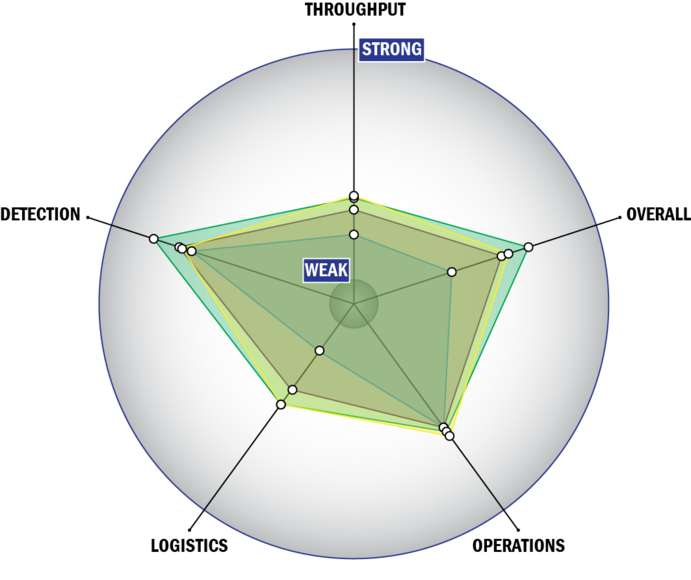
General Description
Ibis personnel invented and developed the Ibis TIGER/T5000 Universal Biosensor, which has been further developed into the Abbott/Ibis PLEX-ID. The biosensor originated from a DARPA program to develop revolutionary sensor technology to meet the needs of the Department of Defense. The technology that resulted from this program is widely held to be a major DARPA success story and the fundamentals of the technology and its validation have been documented in peer-reviewed publications. The Ibis Technology (now commercially available as the PLEX-ID) represents a universal pathogen detection platform capable of identification and strain typing of a broad range of pathogens. The platform employs the PCR (polymerase chain reaction) with primers that amplify relatively conserved regions of the genome(s) present in a sample. Using high-performance electrospray ionization mass spectrometry (ESI-MS), the base composition (i.e., the number of A’s, G’s, C’s, and T’s) of each amplicon is determined and the combination of base compositions from multiple primer pairs is assembled to yield the identity of the organism (s) in the sample. The platform has far-reaching applications in areas such as food safety, biological products screening, and clinical diagnostics. Initial deployments are for the purpose of identification and strain-typing of pathogens associated with biowarfare/bioterrorism events and naturally occurring emerging infectious disease.
Technical Description
In each PCR/ESI-MS assay, multiple pairs of primers are used to amplify carefully selected regions of pathogen genomes; the primer target sites are broadly conserved, but the amplified region carries information on the microbe’s identity in its unique nucleotide base composition. Regions of this nature appear in the DNA that encodes ribosomal RNA and in housekeeping genes that encode essential proteins. Following PCR amplification, a fully automated ESI-MS analysis is performed. This PCR/ESI-MS technology has been successfully used to identify pathogenic bacteria and viruses from a variety of clinical and environmental samples.
Evaluation Criteria
THROUGHPUT:
- Between 60 minutes and 8 hours for detection
- Multiple samples, multiple tests/sample per run
- Less than 32 samples every 2 hours
- The system or device is currently semi-automated
- Device or system is intended for multiple detection assays
- 5 or more solutions, buffer, eluents, and/or reagents
- 5 or more components
- 5-10 minutes is required for set-up
- 6-8 steps are required for detection
LOGISTICS:
- More than a day of training and significant technical skills are required
- Larger than a home dishwasher
- More than 50 kg
- Wired connections are available
- System or device has 220V electrical requirement
OPERATIONS:
- Can be used from 4°C to 37°C
- Components must be frozen (-20°C)
- Device or system has peak performance at normal relative humidity conditions
- Between 6 months and 1 year shelf life
- 5-10 years expected life
- Results can be viewed in real-time
- The system is not capable of autonomy
- The system software is closed and not available for modification
- The system hardware is closed and not available for modification
DETECTION:
- Efforts are underway to achieve 510K clearance
- Efforts are underway to achieve FDA approval
- Less than 100 µL
- Excellent specificity. System has occasional false alarms under certain conditions (<2%)
- 100-1,000 CFU per mL
- 100-1,000 PFU per mL
- Semi-automated spore lysis




 Top Tier
Top Tier Second Tier
Second Tier Third Tier
Third Tier Fourth Tier
Fourth Tier Bottom Tier
Bottom Tier