General Description
Pro-Strips were introduced in 2006 and are certified and designated by the DHS Safety Act. Pro-Strips is the first HHA designed to detect and identify multiple bio-threat agents using one sample. No additional collection kits or expensive readers are required. Each test is packaged in a protective, foil pouch then placed alongside our unique all-in-one buffer collection tube and instruction sheet and Chain of Custody label then sealed in a red mylar pouch which doubles as a re-sealable bio-hazard bag to aid in transportation.
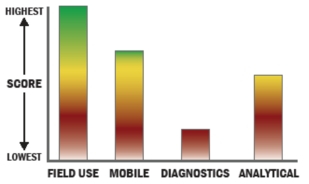
Features include: - Results in as little as 3 minutes
- Excellent detection capabilities
- No cross-reactivity to dozens of near neighbor strains including bacillus thuringensis and bacillus globigii
- No Cross-reactivity to common household substances such as flour, yeast, baby powder, sugar, etc.
- Cost Effective
Technical Description
AdVnt’s Bio-Warfare agent detection systems come in the form of a immunochromatogarphy assay, designed to provide a quick presumptive identification of selected biological warfare agents. Polyclonal antibodies are used because of their superior sensitivity. Monoclonal antibodies are used for their high degree of specificity and sensitivity. These “capture” antibodies are able to grab onto to portion of an antigen with their antigen binding sites
Detector antibodies conjugate to colloidal gold allowing for visualization of the antibody. The capture antibodies are what make up the “T” or test line on the ticket. The collected agents are first introduced into a buffer solution that allows for transport across a wicking pad. As the sample makes its way across the pad it comes into contact with the antispecies line to indicate the test is working properly, then makes contact with the antibodies on the test line. If the antibodies bind with the sample the test line will indicate a positive result.
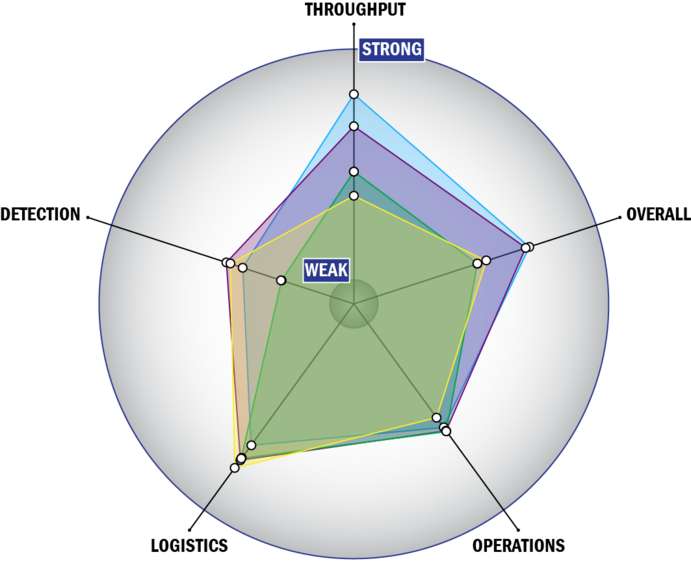
Evaluation Criteria
THROUGHPUT:
- Between 2 and 15 minutes for detection
- 1 sample, <10 tests/sample per run
- The system could be adapted to a semi-automated system with some effort
- Device or system is designed for a single use
- 0-1 solutions, buffer, eluents, and/or reagents
- 0 components
- Less than 5 minutes is required for set-up
- 1-2 steps are required for detection
LOGISTICS:
- Very brief (minutes-hours) training and minimal technical skills
- Approximately the size of a soda can
- Less than 1 kg
- This system is not capable of transmitting data
- There is no electrical requirement
OPERATIONS:
- Can be used from 4°C to 37°C
- Components must be stored at room temperature (27°C)
- Device or system has peak performance at normal relative humidity conditions
- Between 1 to 3 years shelf life
- Results can be viewed in real-time
- The system is not capable of autonomy
DETECTION:
- Possible the system could receive 510K clearance, no current efforts at this time
- Possible the system could receive FDA approval, no current efforts at this time
- Less than 100 µL
- Excellent specificity. System has occasional false alarms under certain conditions (<2%)
- 10,000-100,000 CFU per mL
- 10-100 ng per mL
- Spore lysis not necessary for detection by system




 Top Tier
Top Tier Second Tier
Second Tier Third Tier
Third Tier Fourth Tier
Fourth Tier Bottom Tier
Bottom Tier