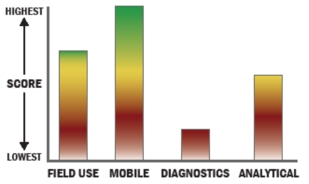
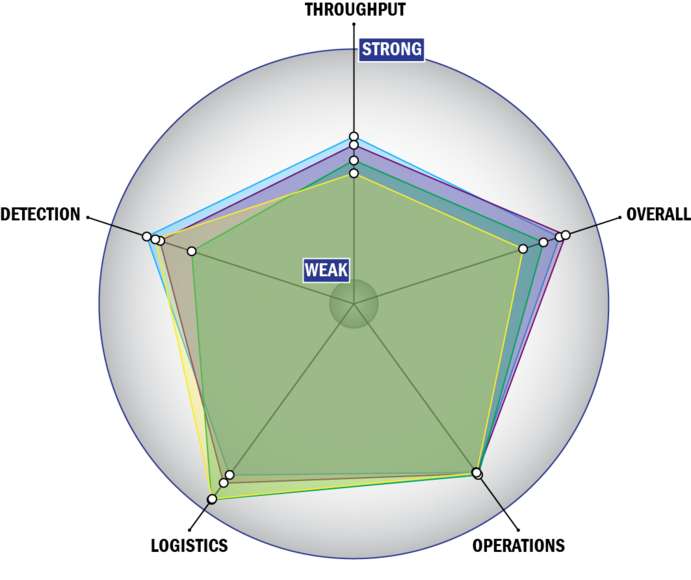
General Description
The BIOSENSOR 2200R biological agent detector is a handheld, portable, on-site instrument for rapid detection, analysis, and identification of biological agents. Unique bioassay technology offers excellent sensitivity and low false positives while offering ease of use in the field. This highly accurate detection method provides rapid measurement of biohazards such as anthrax, ricin, botulism, SEB, and plague. Exclusive five minute time-to-answer allows first responders to make informed critical decisions more rapidly than any other biological agent detector. The BIOSENSOR 2200R employs dynamic surface generation, a patent pending type of immunomagnetic assay detection technology. This technology offers significant advantages over other field-based assay methods by combining the benefits of both the free solution and lateral flow types. The result is more rapid analysis, a user-friendly format, and detector stability within a wide range of climates. Both wet and dry samples may be tested and results are displayed with a simple red (target present) or green (no target present) indication. As tests are nondestructive, samples may be retained as evidence. Single-test, disposable cartridges with on-board reagents have a 16 month shelf life. This instrument is IP 67 certified and permanently housed in a sturdy, light weight fully decon-able Pelican case. Recent research and development has demonstrated that the Biosensor 2200R can identify targets in whole blood and can be utilized as a human diagnostic device.
Technical Description
The BIOSENSOR 2200R employs dynamic surface generation, a patent pending type of immunomagnetic sandwich assay technology. - 1. MIX Sample is mixed with the sensing solution which contains: a magnetic component, a fluorescent component and receptors for the biological agent(s) of interest.
- 2. BIND Sensing materials bind to target during incubation.
- 3. MAGNETIZE All bound and unbound magnetic material is pulled to surface.
- 4. WASH All remaining sensing and non-target material is washed away. False positives are virtually eliminated by removing potential interferents.
- 5. READ Concentrated sample (pellet) is illuminated and emits a signal if target is present.
Evaluation Criteria
THROUGHPUT:
- Between 2 and 15 minutes for detection
- 1 sample, <10 tests/sample per run
- Less than 32 samples every 2 hours
- The system or device is currently semi-automated
- Device or system is intended for multiple detection assays
- 3 solutions, buffer, eluents, and/or reagents
- 1 component
- No set-up of the system is required for set-up
- 6-8 steps are required for detection
LOGISTICS:
- Very brief (minutes-hours) training and minimal technical skills
- Approximately the size of a toaster
- Between 1 and 5 kg
- Wired connections are available
- System or device uses batteries
- 4-8 hours battery life
OPERATIONS:
- Can be used from 4°C to 41°C
- Components must be stored at room temperature (27°C)
- Performance is not influenced by relative humidity
- Between 1 to 3 years shelf life
- Greater than 10 years expected life
- Results can be viewed in real-time
- The system is not capable of autonomy
- The system software is open and available for modification
- The system hardware is open and available for modification
DETECTION:
- Possible the system could receive 510K clearance, no current efforts at this time
- Possible the system could receive FDA approval, no current efforts at this time
- Less than 10 µL
- Superior specificity. System has a false alarm rate approaching zero (~0%)
- 100-1,000 CFU per mL
- 100-1,000 PFU per mL
- Less than 1 ng per mL
- Spore lysis not necessary for detection by system




 Top Tier
Top Tier Second Tier
Second Tier Third Tier
Third Tier Fourth Tier
Fourth Tier Bottom Tier
Bottom Tier