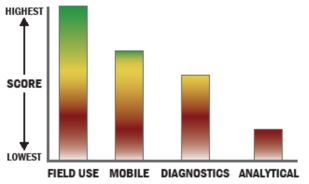
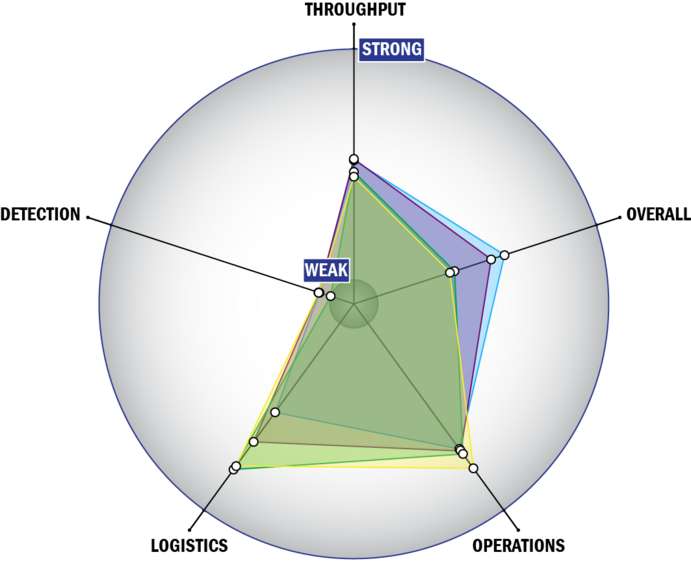
General Description
IntegenX™ created the RapidHIT™ 200 Human DNA Identification System. RapidHIT is the first fully automated sample-to-answer system for STR-based Human Identification (HID). The system is based on the integration of IntegenX’s proprietary, patented and licensed microfluidic technologies and STR-based chemistry. Reagents are provided in single use disposable cartridges are loaded on the system with up to eight buccal swab samples and sample processing is initiated with no further user interaction. The system extracts DNA from the samples and performs STR amplification, electrophoretic separation and software analysis to generate full DNA profiles in about 90 minutes. For the U.S. market, the results data are saved in CODIS-compatible format within the embedded GeneMarker HID® software (SoftGenetics®, LLC). Other data formats will be available as needed.
Technical Description
The core IntegenX technologies include the development, fabrication and programmability of low-cost, microfluidic devices. The key IntegenX technology is the Microscale On-chip Valve (MOVe™). MOVe valves are externally actuated pneumatically-driven miniaturized on-chip valves. MOVe valves can be opened or closed by applying a vacuum or pressure to an air chamber formed by a flexible membrane layer. By combining three or more valves, on-chip MOVe diaphragm micropumps can be formed to transport and process samples. These micropumps control fluidic flow, can readily manipulate volumes from 20 nL to 100 µL, and are excellent in handling paramagnetic beads. MOVe routers can be made from four or more valves to rapidly and effectively mix two nanofluidic streams in seconds, a feature that has been elusive in microfluidics. The second key IntegenX technological breakthrough has been the adaptation of paramagnetic bead technology to work with large volume, “real world” samples, and collapse the targeted contents to a scale suitable for integration with downstream microfluidic devices. A novel feature of the MOVe pumps is their ability to move and capture beads which is not straightforward in other microfluidics devices. The third key IntegenX technology is DevLinkTM software. DevLink is a robust, industrial-strength, distributed instrumentation control platform with an object-oriented application architecture implemented in C# using Microsoft’s .NET Framework. DevLink defines a set of communication and command protocols within a standardized automation architecture. As a result, DevLink forms the basis for both internal breadboard-system development as well as commercial product releases at IntegenX.
Evaluation Criteria
THROUGHPUT:
- Between 60 minutes and 8 hours for detection
- Multiple samples, single tests/sample per run
- Less than 32 samples every 2 hours
- The system or device is currently fully automated
- Device or system is designed for a single use
- 5 or more solutions, buffer, eluents, and/or reagents
- 0 components
- Less than 5 minutes is required for set-up
- Automatic detection
LOGISTICS:
- Very brief (minutes-hours) training and minimal technical skills
- Approximately the size of a carry-on luggage suitcase
- More than 50 kg
- Wireless and wired connections are available
- System or device has 110V electrical requirement
- 1-2 hours battery life
OPERATIONS:
- Can be used from 4°C to 37°C
- Components must be stored at 4°C
- Performance is not influenced by relative humidity
- Between 6 months and 1 year shelf life
- 5-10 years expected life
- Results can be viewed in real-time
- The system could be adapted to a fully autonomous system with some effort
- The system software is closed and not available for modification
- The system hardware is closed and not available for modification
DETECTION:
- Possible the system could receive 510K clearance, no current efforts at this time
- Possible the system could receive FDA approval, no current efforts at this time
- Less than 250 µL
- Superior specificity. System has a false alarm rate approaching zero (~0%)





 Top Tier
Top Tier Second Tier
Second Tier Third Tier
Third Tier Fourth Tier
Fourth Tier Bottom Tier
Bottom Tier