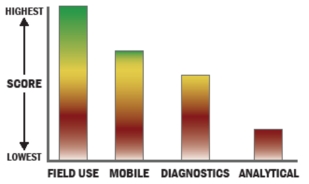
General Description
This rapid, automatic fluorometric assay system is a portable (6.45 kg) 4-channel system for monitoring toxins, viruses, bacteria, spores, fungi and other diverse targets. An extremely reliable third-generation product introduced in 2000, users have found these instruments will operate for two years or more with no breakdowns or leaks, and that they will tolerate debris-laden samples (such as are produced in mailrooms and food processing facilities) - impressive feats for a fully automated wet assay system. Although designed for the military, it works well in the laboratory too.The completely self-contained instrument is the culmination of a careful integration of optics, fluidics, electronics, and software into one compact system for laboratory and field assays. It performs user-defined, multi-step, assay protocols for monitoring fluorescently-labeled immunoassays occurring on the surface of each of the system's four disposable optical waveguide sensors. Toxins and bacteria such as ricin and B. anthracis have been detected at levels below <1.0 ng/mL and 100 CFU/mL, respectively. Four sensors are mounted in a disposable plastic coupon allowing four different pathogens to be detected in a sample; or multiple channels may target the same pathogen to improve statistical certainty. Each coupon may be used for up to 30 assays if negative results are obtained. A bar code on each coupon identifies the type of assay to be run by the instrument and allows very sophisticated assays to be performed by unskilled persons. A computer embedded within the RAPTOR™ performs and controls all steps in the assay procedure.
Technical Description
Research International's Raptor bio-identifier system is based on 'sandwich format' fluoroimmunoassay reactions taking place on the surface of injection molded polystyrene waveguides. All fluidic and optoelectronic steps associated with the assay are performed automatically. In a typical waveguide-based sandwich immunoassay, the waveguide has a monolayer of capture antibody immobilized on its surface. The waveguide is first incubated with sample, washed, then incubated with fluorophore-labeled antibody to form an antibody/antigen/labeled-antibody sandwich. Excitation light is injected into the waveguide and fluorescence emission is collected by a sensitive photodetector that looks down the waveguide axis. The four waveguides are mounted in a small ‘coupon’ that contains a bar code identifier. The onboard computer reads the bar code and automatically performs the proper assay protocol. All reagents and wastes are contained in the portable unit.
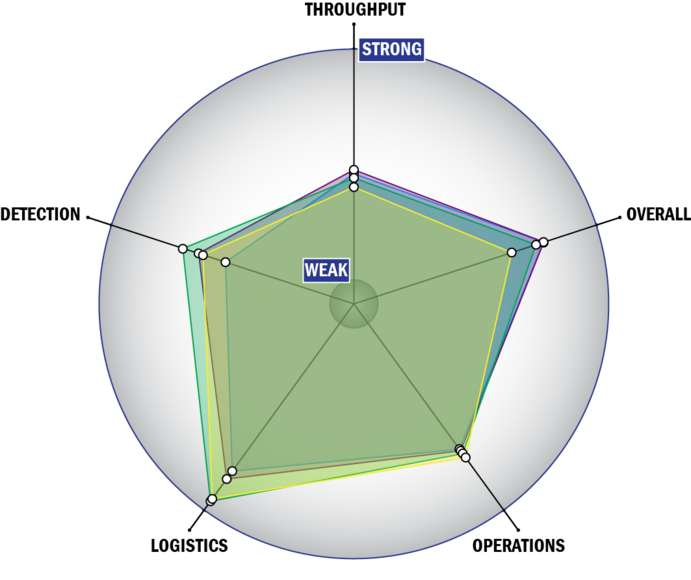
Evaluation Criteria
THROUGHPUT:
- Between 15 and 30 minutes for detection
- 1 sample, <10 tests/sample per run
- Less than 32 samples every 2 hours
- The system or device is currently fully automated
- Device or system is intended for multiple detection assays
- 5 or more solutions, buffer, eluents, and/or reagents
- 1 component
- 10-20 minutes is required for set-up
- 1-2 steps are required for detection
LOGISTICS:
- Very brief (minutes-hours) training and minimal technical skills
- Approximately the size of a toaster
- Between 5 and 25 kg
- Wireless and wired connections are available
- System or device uses batteries
- 4-8 hours battery life
OPERATIONS:
- Can be used from 4°C to 37°C
- Components must be stored at 4°C
- Performance is not influenced by relative humidity
- Between 6 months and 1 year shelf life
- Greater than 10 years expected life
- Results cannot be viewed in real-time
- The system or device is currently fully autonomous
- The system software is closed and not available for modification
- The system hardware is open and available for modification
DETECTION:
- Possible the system could receive 510K clearance, no current efforts at this time
- Possible the system could receive FDA approval, no current efforts at this time
- Greater than 250 µL
- Excellent specificity. System has occasional false alarms under certain conditions (<2%)
- 10,000-100,000 CFU per mL
- Greater than 100,000 PFU per mL
- 1-10 ng per mL
- Spore lysis not necessary for detection by system




 Top Tier
Top Tier Second Tier
Second Tier Third Tier
Third Tier Fourth Tier
Fourth Tier Bottom Tier
Bottom Tier