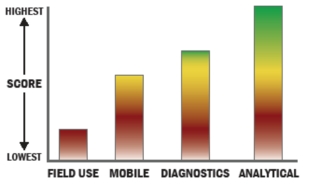
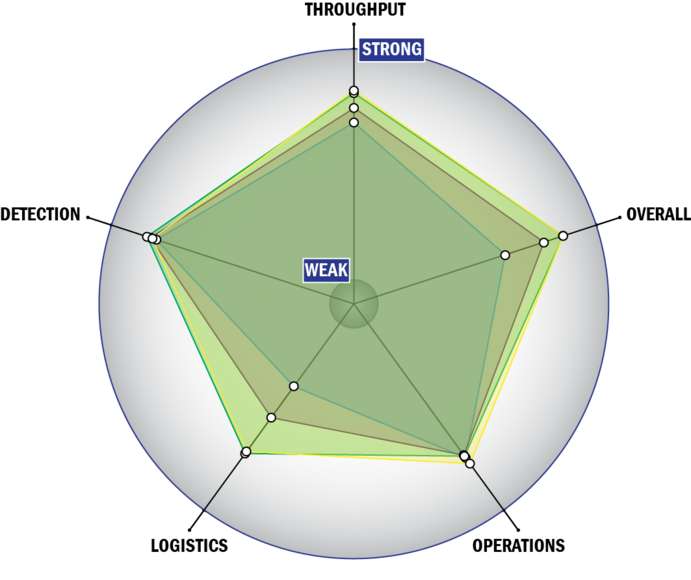
General Description
The Resource Effective BioIdentification System (REBS) -Laboratory Variant is a microbial identification technology that enumerates and identifies microbes using a combination of microscopic imaging and Raman microspectroscopy. The measurement process begins when the system collects particulate material on a solid substrate within a defined region. Next, using standard microscopy techniques, the collected material is imaged at a high magnification, and each field of view is recorded and indexed as the macroscopic collection region is scanned. This iterative process continues until the entire collected region has been scanned. From these images, all particles are located automatically by image processing algorithms, and their morphological properties are used as a first test to discriminate biological particles from non-biological particles. The collection substrate is then moved to bring each particle directly below the microscope objective in order to measure its Raman spectrum. This spectral signature provides accurate discrimination of biological particles from non-biological particles. Moreover, the particle can be identified by matching the measured spectrum to a database of spectra for known materials. Identification is supported only if spectral signatures of the material are available in the spectral database.
Technical Description
Battelle has developed an autonomous micro-Raman spectroscopy technology that enables chemical and biological aerosol identification. The technology incorporates automated sample analysis, single particle Raman spectroscopy, and multivariate signature analysis methods to analyze the chemical and biological materials.
Evaluation Criteria
THROUGHPUT:
- Between 2 and 15 minutes for detection
- Multiple samples, multiple tests/sample per run
- System is continuous and provides real time analysis with no defined tests/samples
- The system or device is currently semi-automated
- Device or system is intended for multiple detection assays
- 0-1 solutions, buffer, eluents, and/or reagents
- 5 or more components
- Less than 5 minutes is required for set-up
- 1-2 steps are required for detection
LOGISTICS:
- An afternoon of training and some technical skills required
- Approximately the size of a carry-on luggage suitcase
- Between 25 and 50 kg
- Wired connections are available
- System or device has 110V electrical requirement
OPERATIONS:
- Can be used from 25°C to 37°C
- Performance is not influenced by relative humidity
- Greater than 3 years shelf life
- 3-5 years expected life
- Results can be viewed in real-time
- The system could easily be adapted into a fully autonomous system
- The system software is open but modification requires licensing
- The system hardware is open but modification requires licensing
DETECTION:
- Efforts are underway to achieve 510K clearance
- Efforts are underway to achieve FDA approval
- Less than 10 µL
- Superior specificity. System has a false alarm rate approaching zero (~0%)
- 1-100 CFU per mL
- 100-1,000 PFU per mL
- 1-10 ng per mL
- Spore lysis not necessary for detection by system
- 1 ppb–1 ppm
- System currently can identify aerosolized chemical agent
- System currently can identify liquid chemical agent





 Top Tier
Top Tier Second Tier
Second Tier Third Tier
Third Tier Fourth Tier
Fourth Tier Bottom Tier
Bottom Tier