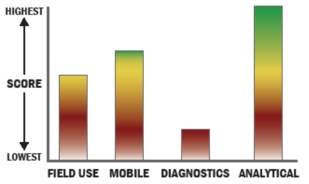
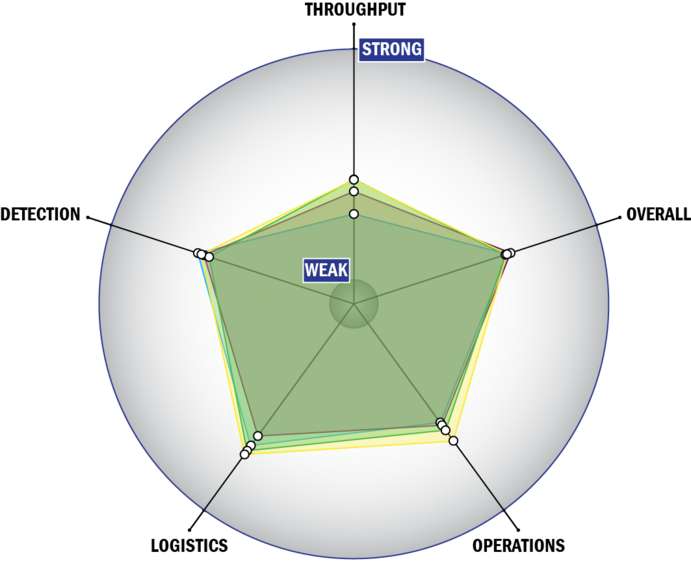
General Description
The Rheonix CARD™ device and EncompassMDx™ instrumentation system were developed to perform fully automated molecular and/or immunoassays. The unique design of the Rheonix CARD™ device allows up to four assays to be simultaneously run on a single disposable device. The Rheonix CARD™ contains all microfluidic channels, reservoirs, pumps, and valves required to perform sophisticated molecular analysis and/or immunoassays at the push of a button. Under the control of our intuitive Encompass MDx™ software, all assay steps are performed automatically once the “raw” sample is applied. No preanalytical steps are required. Due to the ability to perform sophisticated molecular and/or immunoassays without any user intervention, the Rheonix CARD™ platform is well suited for a broad spectrum of applications in either “point-of-care” or central laboratory settings. Thus far, “raw” clinical samples including whole blood, serum plasma, buccal swabs, vaginal swabs and saliva have been successfully tested. Nonclinical samples have included finished drinking water and raw recreational water. Some of the assays that have already been developed include a 20-plex PCR assay that can detect and distinguish 20 clinically relevant HPV subtypes in vaginal swabs, a SNP-based assay that can detect SNPs that are associated with increased sensitivity to warfarin, and a simultaneous immunoassay/PCR-based assay for the direct detection of HIV-1 in human saliva samples. Other assays currently under development include multiplex PCR assays for the detection of sexually transmitted infections, sepsis detection directly from whole blood, EPA-funded efforts to detect Cryptosporidium parvum in drinking water and NSF-funded efforts to detect E. coli and various enterococci in recreational water samples.
Technical Description
Our proprietary and patented lamination process permits us to produce a low cost, plastic CARD™ (Chemistry and Reagent Device) that contains all functionality required to perform a up to four fully automated molecular and/or immunoassays. The portable EncompassMDx™ controller processes one CARD™ (i.e., 4 assays) and the EncompassMDx™ workstation processes up to six CARD™ devices (i.e., 24 assays) per run. In either case, once a raw specimen is introduced into the CARD™ all steps are automatically and seamlessly performed under software control: cell lysis, DNA isolation/purification, multiple-PCR, endpoint detection via an integrated reverse dot blot, and finally output of results. Where necessary, we can also magnetically concentrate either cells or nucleic acids.
Evaluation Criteria
THROUGHPUT:
- Between 60 minutes and 8 hours for detection
- Multiple samples, multiple tests/sample per run
- The system or device is currently fully automated
- Device or system is designed for a single use
- 5 or more solutions, buffer, eluents, and/or reagents
- 4 components
- Less than 5 minutes is required for set-up
- 1-2 steps are required for detection
LOGISTICS:
- Very brief (minutes-hours) training and minimal technical skills
- Approximately the size of a carry-on luggage suitcase
- Between 5 and 25 kg
- Satellite and wired connections are available
- System or device has 110V electrical requirement
- 2-4 hours battery life
OPERATIONS:
- Can be used from 25°C to 37°C
- Performance is not influenced by relative humidity
- Between 6 months and 1 year shelf life
- Results cannot be viewed in real-time
- The system or device is currently fully autonomous
- The system software is closed and not available for modification
- The system hardware is closed and not available for modification
DETECTION:
- Possible the system could receive 510K clearance, no current efforts at this time
- Possible the system could receive FDA approval, no current efforts at this time
- Less than 10 µL
- Good specificity. System has a consistently low level of false alarms (2-5%)
- 1-100 CFU per mL
- 1-100 PFU per mL
- Add on capability that is full or semi-automated for spore lysis




 Top Tier
Top Tier Second Tier
Second Tier Third Tier
Third Tier Fourth Tier
Fourth Tier Bottom Tier
Bottom Tier