General Description
The system consists of a low cost scanning system (hardware, software and computer included) that is used to measure antibodies to pathogens (viral or bacterial) in patient (human, monkey, rodent, etc.) blood or sera. Pathogen panels for screening are available off-the-shelf or can be easily customized to meet customer needs.
Technical Description
The ultra-senstive detection system (2-10 times > fluorescence is based on gold nano-particles conjugated to species specific antibodies which direct the deposition of silver particles in order to visualize the pathogen spot and identify the infectious organism.
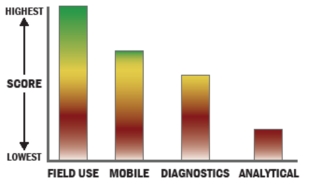
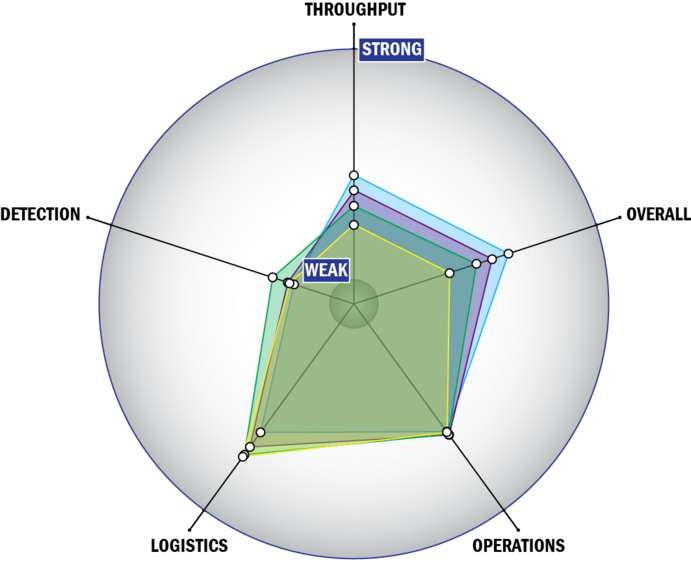
Evaluation Criteria
THROUGHPUT:
- Between 60 minutes and 8 hours for detection
- 1 sample, >10 tests/sample per run
- 95-32 samples every 2 hours
- The system could be adapted to a fully automated system with some effort
- Device or system is intended for multiple detection assays
- 4 solutions, buffer, eluents, and/or reagents
- 1 component
- Less than 5 minutes is required for set-up
- 9-12 steps are required for detection
LOGISTICS:
- Very brief (minutes-hours) training and minimal technical skills
- Approximately the size of a carry-on luggage suitcase
- Between 5 and 25 kg
- Satellite, wireless and wired connections are available
- System or device has 110V electrical requirement
OPERATIONS:
- Can be used from 4°C to 37°C
- Components must be stored at 4°C
- Performance is not influenced by relative humidity
- Between 6 months and 1 year shelf life
- 5-10 years expected life
- Results cannot be viewed in real-time
- The system is not capable of autonomy
- The system software is open but modification requires licensing
- The system hardware is open and available for modification
DETECTION:
- Possible the system could receive 510K clearance, no current efforts at this time
- Possible the system could receive FDA approval, no current efforts at this time
- Less than 10 µL
- Excellent specificity. System has occasional false alarms under certain conditions (<2%)




 Top Tier
Top Tier Second Tier
Second Tier Third Tier
Third Tier Fourth Tier
Fourth Tier Bottom Tier
Bottom Tier