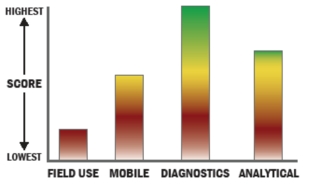
General Description
The TessArray® RPM-Flu assay simultaneously detects and definitively identifies more than 35 distinct classes of respiratory agents, both viral and bacterial, in a single assay in less than 24 hours. The assay generates agent-specific genomic sequence information as the output, and can easily discriminate between known, unknown, emerging, and deliberately altered pathogens to the subtype and/or strain level. The assay is designed for use by centralized testing laboratories to detect respiratory agents in throat swabs, nasal washes, Broncho alveolar lavages, and other clinical matrices.
Technical Description
The TessArray RPM-Flu assay is based on resequencing microarray technology that runs on an Affymetrix® GeneChip® 3000 System. Genomic material (both RNA and DNA) extracted from the sample is amplified using locus-specific primers in highly multiplexed reactions (30-40 primer pairs/reaction) using a relaxed amplification strategy to maximize assay sensitivity. Amplified material is hybridized to the resequencing microarray under stringent conditions to maximize assay specificity. The TessArae Gene Cipher algorithm analyzes the resulting sequence data to detect and identify the agents present in the sample. RPM-Flu is the only assay that simultaneously maximizes both sensitivity and specificity.
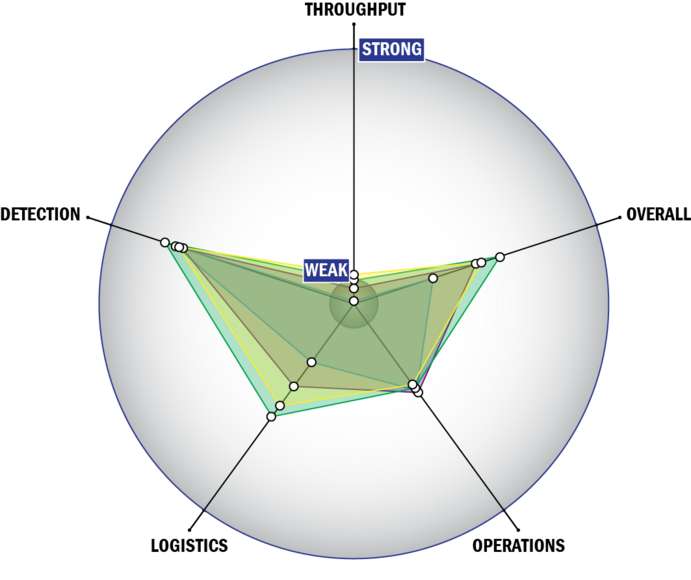
Evaluation Criteria
THROUGHPUT:
- Greater than 8 hours for detection
- Multiple samples, single tests/sample per run
- Less than 32 samples every 2 hours
- The system could be adapted to a semi-automated system with some effort
- Device or system is designed for a single use
- 5 or more solutions, buffer, eluents, and/or reagents
- 5 or more components
- 10-20 minutes is required for set-up
- Greater than 12 steps are required for detection
LOGISTICS:
- More than a day of training and significant technical skills are required
- Larger than a home dishwasher
- More than 50 kg
- Wireless and wired connections are available
- System or device has 110V electrical requirement
OPERATIONS:
- Can be used from 25°C to 37°C
- Components must be frozen (-20°C)
- Performance is not influenced by relative humidity
- Between 6 months and 1 year shelf life
- 5-10 years expected life
- Results cannot be viewed in real-time
- The system is not capable of autonomy
- The system software is closed and not available for modification
- The system hardware is closed and not available for modification
DETECTION:
- Efforts are underway to achieve 510K clearance
- System currently has FDA approval
- Less than 50 µL
- Superior specificity. System has a false alarm rate approaching zero (~0%)
- 1-100 CFU per mL
- 1-100 PFU per mL
- Manual kit not integrated with the system handles spore lysis




 Top Tier
Top Tier Second Tier
Second Tier Third Tier
Third Tier Fourth Tier
Fourth Tier Bottom Tier
Bottom Tier