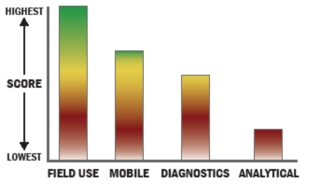
General Description
The Test-mate ChE is a complete, self-contained and portable cholinesterase testing system. The system requires only 10µL for each blood test, which may be conveniently obtained from a fingerstick sample. The entire assay may be completed in less than 4 minutes, facilitating the rapid evaluation of poisoning status. The small size (11” x 7” x 10”) and weight (10 pounds) allows the unit to be easily transported between test sites.
Technical Description
The Test-mate ChE reagents are based on the Ellman method. Acetylthiocholine (AcTC) or butyrylthiocholine (BuTC) is hydrolyzed by AChE or PChE, respectively, producing carboxylic acid and thiocholine which reacts with the Ellman reagent (DTNB, dithionitrobenzoic acid) to form a yellow color which is measured spectrophotometrically at 450nm. The rate of color formation is proportional to the amount of either AChE or PChE.
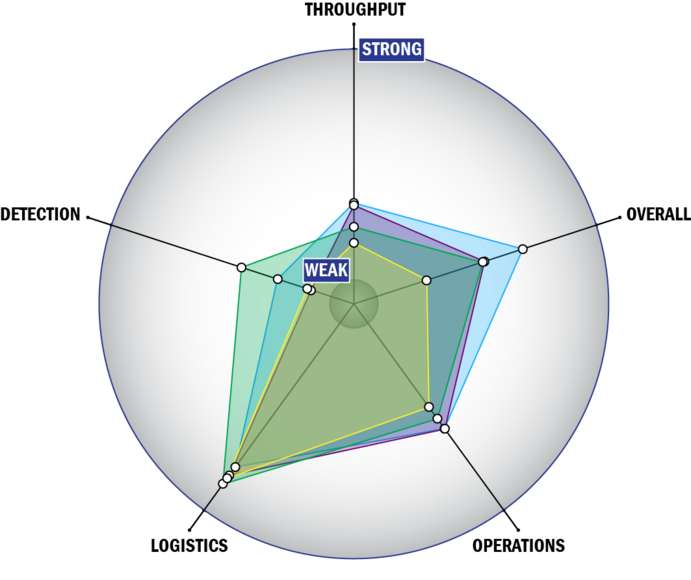
Evaluation Criteria
THROUGHPUT:
- Between 2 and 15 minutes for detection
- 1 sample, single test/sample per run
- Less than 32 samples every 2 hours
- The system or approach is not amenable to full or semi-automation
- Device or system is intended for multiple detection assays
- 3 solutions, buffer, eluents, and/or reagents
- 4 components
- Less than 5 minutes is required for set-up
- 9-12 steps are required for detection
LOGISTICS:
- An afternoon of training and some technical skills required
- Approximately the size of a toaster
- Between 1 and 5 kg
- System or device uses batteries
- 4-8 hours battery life
OPERATIONS:
- Can be used from 4°C to 37°C
- Components must be stored at room temperature (27°C)
- Performance is not influenced by relative humidity
- Between 1 to 3 years shelf life
- 5-10 years expected life
- Results cannot be viewed in real-time
- The system is not capable of autonomy
- The system software is closed and not available for modification
- The system hardware is closed and not available for modification
DETECTION:
- System currently has 510k clearance
- System currently has FDA approval
- Less than 50 µL
- Good specificity. System has a consistently low level of false alarms (2-5%)




 Top Tier
Top Tier Second Tier
Second Tier Third Tier
Third Tier Fourth Tier
Fourth Tier Bottom Tier
Bottom Tier