General Description
The BioMark Family of systems are designed for high throughput real-time PCR. The system is designed for research use only.
Technical Description
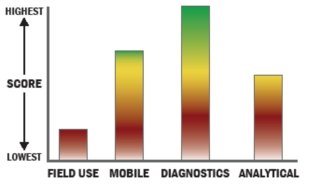
The BioMark Family of Real-Time PCR systems enables high throughput real time PCR with nanoliter sized reaction volumes through the use of microfluidics. The microfluidic architecture does the work of combining samples and assays into 9,216 simultaneous PCR reactions, which is 24-fold more data than that produced by a 384-well plate.
Evaluation Criteria
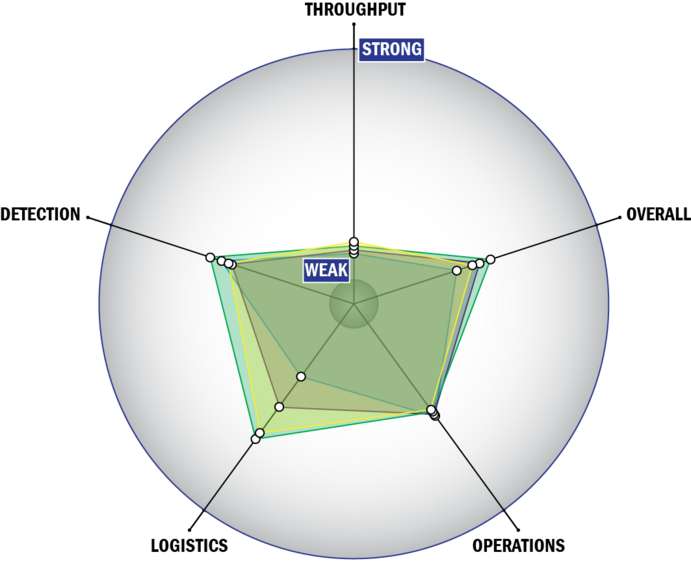
THROUGHPUT:
- Between 60 minutes and 8 hours for detection
- Multiple samples, multiple tests/sample per run
- The system could be adapted to a semi-automated system with some effort
- Device or system is intended for multiple detection assays
- 5 or more solutions, buffer, eluents, and/or reagents
- 3 components
- Greater than 20 minutes is required for set-up
- Greater than 12 steps are required for detection
LOGISTICS:
- An afternoon of training and some technical skills required
- Larger than a home dishwasher
- More than 50 kg
- Wired connections are available
- System or device has 110V electrical requirement
OPERATIONS:
- Can be used from 25°C to 37°C
- Components must be stored at room temperature (27°C)
- Device or system has peak performance at normal relative humidity conditions
- Between 6 months and 1 year shelf life
- 5-10 years expected life
- Results can be viewed in real-time
- The system is not capable of autonomy
- The system software is closed and not available for modification
- The system hardware is closed and not available for modification
DETECTION:
- Possible the system could receive 510K clearance, no current efforts at this time
- Possible the system could receive FDA approval, no current efforts at this time
- Less than 10 µL
- 1-100 CFU per mL
- 1-100 PFU per mL
- Manual kit not integrated with the system handles spore lysis




 Top Tier
Top Tier Second Tier
Second Tier Third Tier
Third Tier Fourth Tier
Fourth Tier Bottom Tier
Bottom Tier