General Description
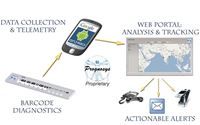
The AIDA™ system is comprised of a multiple pathogens and toxins diagnostic strip, a smart telephone for reading, analyzing and telemetry of the results indicated on the diagnostic strip, and a secure (encrypted) web portal for capturing test data, tracking results, prognosis, decision support, and mapping of identified pathogens and toxins anywhere on the globe. AIDA™ can be used to detect as many as ten pathogens, toxins, chemical threats, and physical conditions (such as temperature, time, and vibration) simultaneously. AIDA™ devices can be configured as qualitative devices or fully quantitative systems with a limit of detection of about 50 ng/mL or 104 CFU, specificity of 95% or better, coefficient of variation of 5% or less, and detection time of less than 10 minutes for all indications on the strip. Prognosys has recently developed multiple pathogen cassettes for B. anthracis spores and PA, C. botulinum toxin, Yersenia pestis, SEB and Ricin, and a number of other diagnostics such as enteric diseases (V. colerae O1, O139, E-coli O157, Salmonella), cardiovascular disease markers (CRP, cTnI, TpP), and a number of kidney disease markers. Developed by Prognosys, LLC as point-of-care diagnostic system, AIDA™ can be used by un-trained individuals at home, in the field, and points of care, without the need for instruments, reagents, and processing. AIDA™ is highly configurable not only for biological threats and medical applications, but also for supply chain tracking and security, tamper detection, and organizational planning and decision support.
Technical Description
Commercially available lateral flow immuno-assays using colloidal gold labeled antibodies, incorporated into barcode diagnostic devices.
Evaluation Criteria
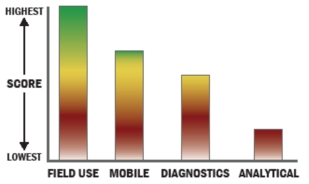
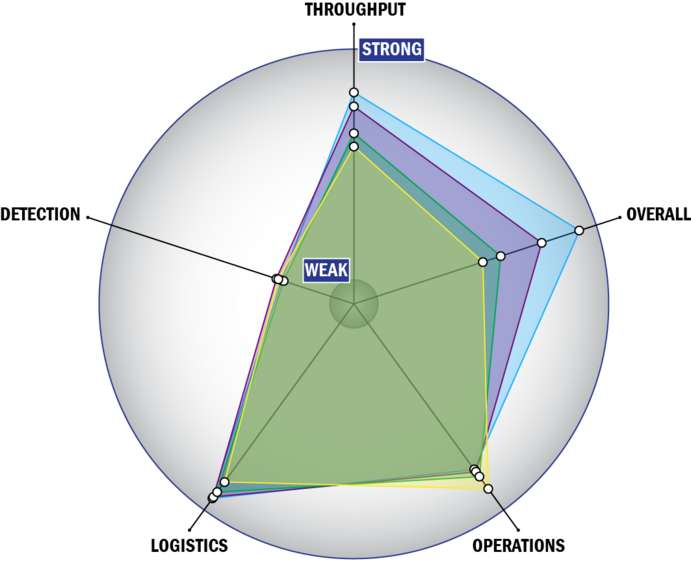
THROUGHPUT:
- 2 minutes or less for detection
- 1 sample, >10 tests/sample per run
- The system could easily be adapted into a fully automated system
- Device or system is intended for multiple detection assays
- 0-1 solutions, buffer, eluents, and/or reagents
- 1 component
- No set-up is required for setup
- 1-2 steps are required for detection
LOGISTICS:
- An afternoon of training and some technical skills required
- Approximately the size of a soda can
- Less than 1 kg
- Satellite, wireless and wired connections are available
- System or device uses batteries
- 2-4 hours battery life
OPERATIONS:
- Can be used from 4°C to 41°C
- Components must be stored at 4°C
- Device or system has peak performance at normal relative humidity conditions
- Between 1 to 3 years shelf life
- 1-3 years expected life
- Results can be viewed in real-time
- The system could easily be adapted into a fully autonomous system
- The system software is open but modification requires licensing
- The system hardware is open but modification requires licensing
DETECTION:
- Possible the system could receive 510K clearance, no current efforts at this time
- Less than 50 µL
- 10,000-100,000 CFU per mL
- 10,000-100,000 PFU per mL
- Greater than 10,000 ng per mL
- Manual kit not integrated with the system handles spore lysis
- > 1x10-3 mg/m3
- 1 ppb – 1 ppm



 Top Tier
Top Tier Second Tier
Second Tier Third Tier
Third Tier Fourth Tier
Fourth Tier Bottom Tier
Bottom Tier