General Description
The Akonni TruArray is a portable microarray in vitro diagnostic device employing a dual nucleic acid and immunoassay test methodology to increase diagnostic confidence limits of rare and emerging infectious diseases. The platform includes sample preparation, reagents, microarray detection, and all hardware and software to run an end-to-end protocol. Because of the unique attributes of gel element arrays and the disposable fluidic cartridge, Akonni can offer a user the ability to analyze for hundreds of genetic- or immuno-based disease traits in one, rapid, simple to use platform. The state-of-the-art technology can easily be adapted to custom DHHS or DOD applications and can be quickly re-configured for new or emerging infectious disease targets. Military, EMT and medical personnel could use the portable device, with minimal training and expense, to identify the presence of pathogens.
Technical Description
The platform employs a patented sample preparation technology (TruTip) for rapid (<4 mins) extraction, purification and concentration of nucleic acid from clinical and environmental samples. The core gel-drop microarray technology was exclusively licensed from Argonne National Laboratory whom led the development of the technology throughout the 1990s under funding from the Human Genome Program and from DOD’s DARPA biodefense program. The biological assay (PCR and immunocapture) was developed by USAMRIID and ported over to Akonni’s microarray chip under a DTRA program. Subsequently, Akonni obtained a license from USAMRIID and its affiliates, to the nucleic acid sequences, primers and probes.
Evaluation Criteria
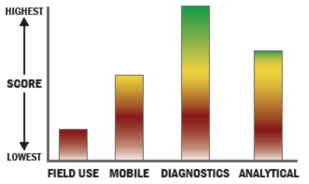
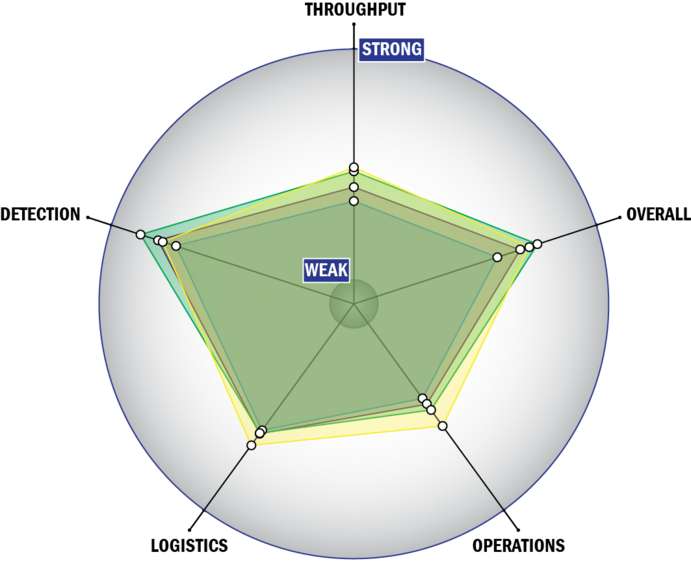
THROUGHPUT:
- Between 15 and 30 minutes for detection
- 1 sample, >10 tests/sample per run
- 749-350 samples every 2 hours
- The system or device is currently semi-automated
- Device or system is intended for multiple detection assays
- 4 solutions, buffer, eluents, and/or reagents
- 3 components
- 5-10 minutes is required for set-up
- 9-12 steps are required for detection
LOGISTICS:
- An afternoon of training and some technical skills required
- Approximately the size of a toaster
- Between 5 and 25 kg
- Wireless and wired connections are available
- System or device has 110V electrical requirement
- 1-2 hours battery life
OPERATIONS:
- Can be used from 4°C to 37°C
- Components must be frozen (-20°C)
- Between 6 months and 1 year shelf life
- Results cannot be viewed in real-time
- The system could be adapted to a fully autonomous system with significant effort
- The system software is open but modification requires licensing
- The system hardware is open but modification requires licensing
DETECTION:
- Efforts are underway to achieve 510K clearance
- System currently has FDA approval
- Greater than 250 µL
- 100-1,000 CFU per mL
- 100-1,000 PFU per mL
- 1-10 ng per mL
- Semi-automated spore lysis

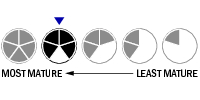



 Top Tier
Top Tier Second Tier
Second Tier Third Tier
Third Tier Fourth Tier
Fourth Tier Bottom Tier
Bottom Tier