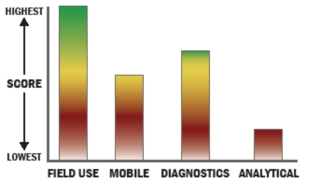
General Description
The ViriChip System is a technology platform for direct detection and characterization of viral particles rather than nucleic acid or antigens. The affinity substrate used is termed the ‘ViriChip’. The ViriChip contains type-specific antibody or ligand domains capable of capturing intact infectious viruses. The integration of AFM with the ViriChip has resulted in the development of an atomic force microscopy–immunoassay (AFMIA). The AFMIA combines two key features: specificity determined by antibody capture, and a label-free AFM readout that offers the additional benefit of providing topographical/morphological information to corroborate affinity-based virus identification. Principal benefits of AFM readout include size of apparatus (hand held) and multiplexing for multiple viruses on single ViriChips. The ViriChip System is versatile being designed for: - 1. Use in field operations
- 2. Use in laboratory analysis
- 3. Use in remote testing by UAV or other vehicles
- 4. Use in clinical diagnostic laboratories.
Technical Description
The ViriChip System consists of a functionalized chip containing 10 micrometer domains of specific capture ligands (antibodies, aptamers, etc.) arrayed in multiplexed format. The specific ligands are qualified to capture intact virus particles, virus-like particles, or sub-virion structures existing on the outer surface of the virus. The chip, assembled into a microfluidics cartridge, receives and analyzes fluids for virus capture. Processing and readout by atomic force microscopy are automated. Analysis software is coordinated with data collection. Data and analysis transmission is designed for existing communication mechanisms. The System will detect and identify biothreat viral agents collected from water, air, foods, surfaces, and body fluids.
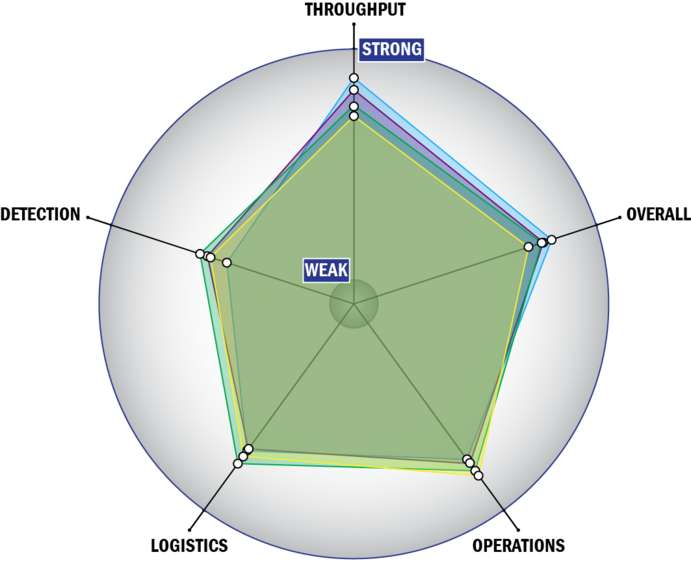
Evaluation Criteria
THROUGHPUT:
- Between 15 and 30 minutes for detection
- Multiple samples, multiple tests/sample per run
- Greater than 750 samples every 2 hours
- The system could be adapted to a fully automated system with some effort
- Device or system is intended for multiple detection assays
- 0-1 solutions, buffer, eluents, and/or reagents
- 1 component
- Less than 5 minutes is required for set-up
- 1-2 steps are required for detection
LOGISTICS:
- Very brief (minutes-hours) training and minimal technical skills
- Approximately the size of a toaster
- Between 5 and 25 kg
- System or device uses batteries
- 4-8 hours battery life
OPERATIONS:
- Can be used from 4°C to 41°C
- Components must be stored at 4°C
- Performance is not influenced by relative humidity
- Between 6 months and 1 year shelf life
- 5-10 years expected life
- Results can be viewed in real-time
- The system could easily be adapted into a fully autonomous system
- The system software is open but modification requires licensing
- The system hardware is open but modification requires licensing
DETECTION:
- Possible the system could receive 510K clearance, no current efforts at this time
- Possible the system could receive FDA approval, no current efforts at this time
- Less than 10 µL
- Excellent specificity. System has occasional false alarms under certain conditions (<2%)
- 1-100 PFU per mL




 Top Tier
Top Tier Second Tier
Second Tier Third Tier
Third Tier Fourth Tier
Fourth Tier Bottom Tier
Bottom Tier