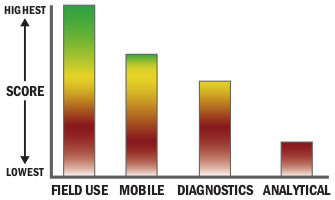
General Description
The Biothreat Detection IMASS™ is used to directly sample powders, surfaces or liquids using an integrated sponge, then eight immunoassay strips are run simultaneously from the sample giving results for eight biothreat agents in 15 minutes from a single sample.
Technical Description
Lateral Flow Immunoassay. A test strip, usually made of nitrocellulose, includes signal and capture reagents specific to the biothreat target of interest. Antibody is striped onto the membrane (capture reagent) and Antibody is attached (conjugated) onto signal reagent ( gold particle). Sample is added at one end of the stick, and runs along the test laterally, if the target is present a line, the gold capture agent will bind to the agent and the agent will bind to the antibody striped on the membrane to form a coloured line.
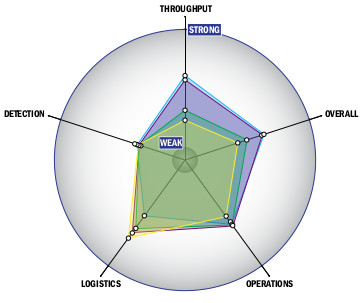
Evaluation Criteria
THROUGHPUT:
- Between 2 and 15 minutes for detection
- 1 sample, <10 tests/ sample per run
- Less than 32 samples every 2 hour
- The system or approach is not amenable to full or semiautomation
- Device or system is designed for a single use
- 0-1 solutions, buffer, eluents, and/or reagents
- 0 components
- No set-up of the system is required
- 3-5 steps are required for detection
LOGISTICS:
- Very brief (minutes-hours) training and minimal technical skills
- Approximately the size of a soda can
- Less than 1 kg
- This system is not capable of transmitting data
- System or device requires multiple outlets or a dedicated circuit breaker
OPERATIONS:
- Can be used from 4°C to 37°C
- This system does not require consumable components
- Device or system has peak performance at normal relative humidity conditions
- Between 1 to 3 years shelf life
- This system or device is single use and does not have an expected life
- The system is not capable of autonomy
- The system does not employ any software
- The system is single use or this question does not apply to this device
DETECTION:
- Not possible for the system to achieve 510K clearance
- Not possible for the system to achieve FDA approval
- Less than 50 µl
- Good specificity. System has a consistently low level of false alarms (2-5%)
- 10,000-100,000 CFU per ml
- 1-10 ng per ml
- Spore lysis not necessary for detection by system




 Top Tier
Top Tier Second Tier
Second Tier Third Tier
Third Tier Fourth Tier
Fourth Tier Bottom Tier
Bottom Tier