General Description
The Applied Biosystems® 7500 Fast Dx Real-Time PCR Instrument is a real-time PCR instrument used in conjunction with FDA-cleared assays for diagnostic detection of genetic material. Available assays include the CDC's H1N1 flu virus and Dengue assays.
Technical Description
The Applied Biosystems® 7500 Fast Dx Real-Time PCR Instrument, with SDS v1.4 Software, is a 96-well, 5-color real-time PCR instrument available for in vitro diagnostic use in the laboratory that employs real-time TaqMan® polymerase chain reaction amplification of genes on a peltier-based thermocycling apparatus.
Notes:
This device is used by the DoD and CDC LRN laboratories for real-time PCR analysis.
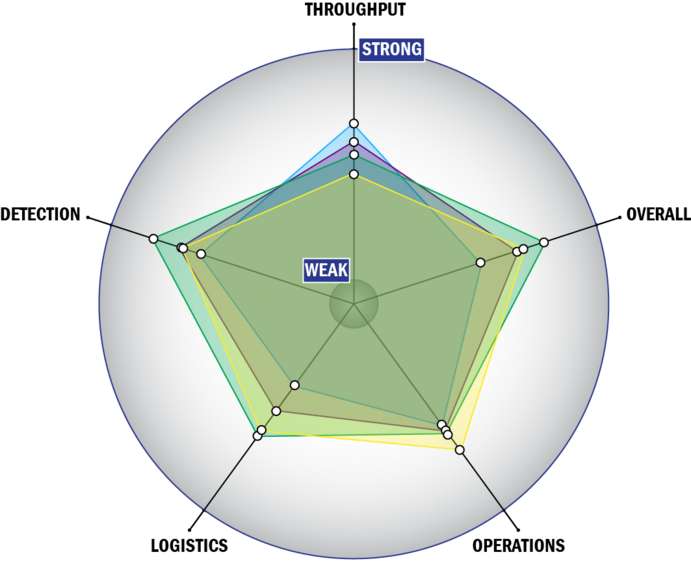
Evaluation Criteria
THROUGHPUT:
- Between 30 and 60 minutes for detection
- Multiple samples, multiple tests/sample per run
- 349-96 samples every 2 hours
- The system could be adapted to a semi-automated system with some effort
- Device or system is intended for multiple detection assays
- 0-1 solutions, buffer, eluents, and/or reagents
- 3 components
- No set-up of the system is required
- 3-5 steps are required for detection
LOGISTICS:
- A day of training and technical skills are required
- Approximately the size of a carry-on luggage suitcase
- Between 25 and 50 kg
- Wireless and wired connections are available
- System or device has 110V electrical requirement
OPERATIONS:
- Can be used from 25°C to 37°C
- Components must be frozen (-20°C)
- Performance is not influenced by relative humidity
- Between 6 months and 1 year shelf life
- 5-10 years expected life
- Results can be viewed in real-time
- The system is not capable of autonomy
- The system software is closed and not available for modification
- The system hardware is closed and not available for modification
DETECTION:
- System currently has 510k clearance
- System currently has FDA approval
- Less than 50 µL
- 1-100 CFU per mL
- 1-100 PFU per mL
- Manual kit not integrated with the system handles spore lysis




 Top Tier
Top Tier Second Tier
Second Tier Third Tier
Third Tier Fourth Tier
Fourth Tier Bottom Tier
Bottom Tier