General Description
BioCheck® is a field deployable test to screen unknown powders for the presence of biological material. Designed for ease of use, high sensitivity and fast result delivery, BioCheck functions as presumptive test for an initial biological screen and can deliver a result in under five minutes. Normally, the BioCheck test will be performed prior to any pathogen specific tests are undertaken to determine whether there is justification for the time and cost for other tests to be performed. As a highly sensitive field test for positive protein identification, BioCheck was designed specifically to quickly rule out the presence of any biological pathogen and quickly allow decisions to be made confidently regarding the next steps for additional testing in a white powder /suspicious powder decision matrix or SOP. BioCheck test kits do not require any instrumentation, have no power requirements, and are rugged. Each kit is individually packaged with a very small form factor and are completely disposable after use. Extensively tested and validated, BioCheck has been shown to detect minute amounts of biological material when testing unknown powders. Recent US Army ECBC testing showed sensitivity to as little as 100 µg of Ricin and 1 x 107 cfu of B. anthracis spores.
Technical Description
BioCheck is a patented field test that functions as a colormetric assay that detects protein in solid materials and gives a color change when even minute amounts of protein are present. Specifically optimized in sensitivity and specificity its LOD is calibrated to give a high positive predictive value.
Notes:
General suspicious powder test kit used by first responders.
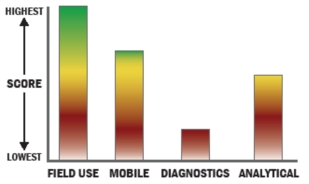
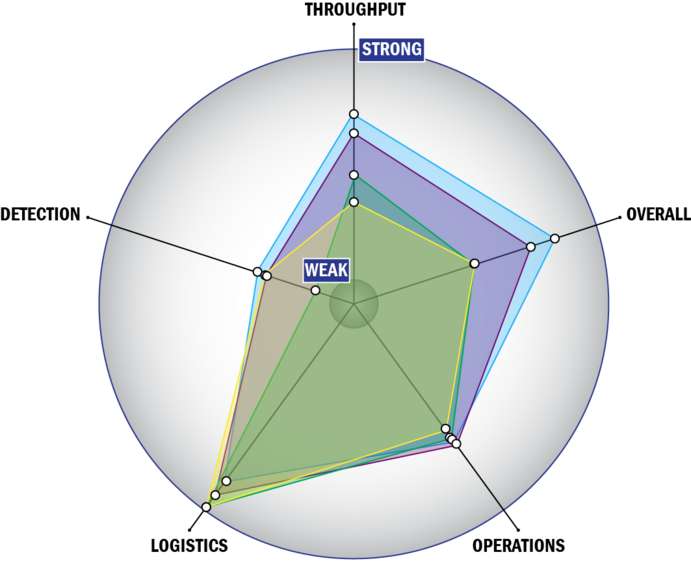
Evaluation Criteria
THROUGHPUT:
- Between 2 and 15 minutes for detection
- 1 sample, single test/sample per run
- 95-32 samples every 2 hours
- The system or approach is not amenable to full or semi-automation
- Device or system is designed for a single use
- 2 solutions, buffer, eluents, and/or reagents
- 1 component
- No set-up of the system is required
- 1-2 steps are required for detection
LOGISTICS:
- Very brief (minutes-hours) training and minimal technical skills
- Approximately the size of a soda can
- Less than 1 kg
- This system is not capable of transmitting data
- 4–8 Hours battery life
OPERATIONS:
- Can be used from 4°C to 41°C
- Components must be stored at room temperature (27°C)
- Performance is not influenced by relative humidity
- Between 1 to 3 years shelf life
- Results can be viewed in real-time
- The system is not capable of autonomy
DETECTION:
- Not possible for the system to achieve 510K clearance
- Not possible for the system to achieve FDA approval
- This system does not test liquids
- Superior specificity. System has a false alarm rate approaching zero (~0%)
- Greater than 100,000 CFU per mL
- Greater than 100,000 PFU per mL
- 1,000-10,000 ng per mL
- Spore lysis not necessary for detection by system



 Top Tier
Top Tier Second Tier
Second Tier Third Tier
Third Tier Fourth Tier
Fourth Tier Bottom Tier
Bottom Tier