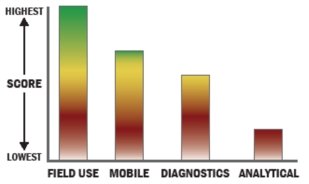
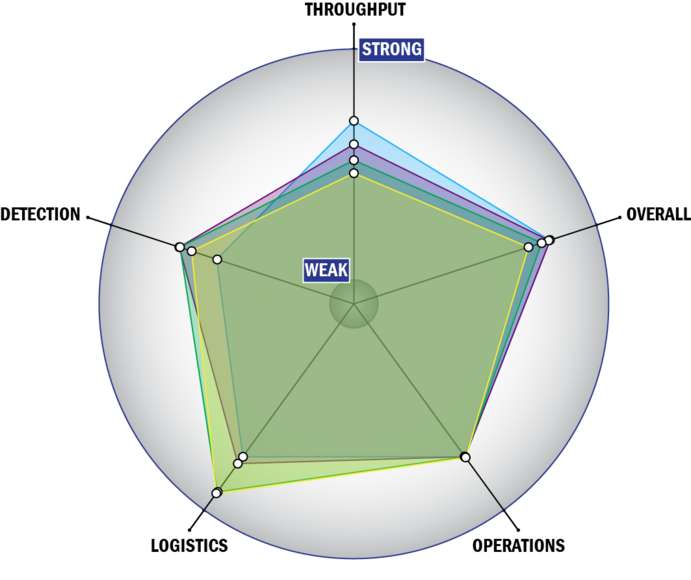
General Description
The BioHawk is a portable 8-channel bioassay system with automated sample collection capability that is suitable for high-sensitivity monitoring of biological agents. BioHawk can monitor surrounding air for aerosol threats with its built-in air sampler, and periodically transfer a liquid concentrate from the air sampler to the bioidentifier. Assay results are typically available in 20 minutes. The biodetector portion of the instrument will also accept fluids for analysis from other sample collection systems, or will process liquid samples loaded into the instrument manually. Bioassays are performed within a small disposable assay coupon that may be used for up to 10 assays. This capability can substantially reduce life cycle cost. Assay results may be transmitted to users through the touch panel LCD display, an audible alarm, by wireless or RS-232 link to personnel at a remote location. System operation may be remotely controlled in real time virtually.
Technical Description
The BioHawk is an integration of Research International's proven SASS 2300 air sampler technology with an 8-channel bio-identifier. In operation, the SASS 2300 samples air continuously and transfers particulates into a secondary water phase. The water samples are periodically transferred from the air sampler to the bio-identifier. The sample is used for analysis and then may be saved as a confirmatory sample. The BioHawk automatically performs analysis for up to eight agents on the disposable assay coupon. The coupons may be reused up to 10 times before replacement is necessary. Assays are based on 'sandwich format' fluoroimmunoassay reactions taking place on the surface of injection molded polystyrene waveguides. All fluidic and optoelectronic steps associated with the assay are performed automatically.
Evaluation Criteria
THROUGHPUT:
- Between 15 and 30 minutes for detection
- 1 sample, <10 tests/sample per run
- 95-32 samples every 2 hours
- The system or device is currently fully automated
- Device or system is intended for multiple detection assays
- 2 solutions, buffer, eluents, and/or reagents
- 0 components
- Greater than 20 minutes is required for set-up
- Automatic detection
LOGISTICS:
- Very brief (minutes-hours) training and minimal technical skills
- Approximately the size of a carry-on luggage suitcase
- Between 5 and 25 kg
- Wireless and wired connections are available
- System or device uses batteries
- 4-8 hours battery life
OPERATIONS:
- Can be used from 4°C to 41°C
- Components must be stored at room temperature (27°C)
- Performance is not influenced by relative humidity
- Between 6 months and 1 year shelf life
- Greater than 10 years expected life
- Results cannot be viewed in real-time
- The system or device is currently fully autonomous
- The system software is closed and not available for modification
- The system hardware is open and available for modification
DETECTION:
- Possible the system could receive 510K clearance, no current efforts at this time
- Possible the system could receive FDA approval, no current efforts at this time
- Greater than 250 µL
- Excellent specificity. System has occasional false alarms under certain conditions (<2%)
- 10,000-100,000 CFU per mL
- Greater than 100,000 PFU per mL
- 1-10 ng per mL
- Spore lysis not necessary for detection by system




 Top Tier
Top Tier Second Tier
Second Tier Third Tier
Third Tier Fourth Tier
Fourth Tier Bottom Tier
Bottom Tier