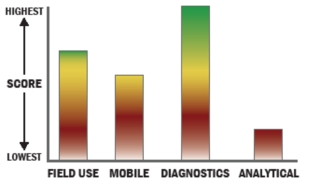
General Description
Fully automated, random access multiplex system designed for Autoimmune and Infectious disease testing in human serum or plasma. The system is designed to up to 22 specific analytes per assay panel. The system is utilized in diagnostic testing in hospital laboratories.
Technical Description
BioPlex 2200 Immunoassay use heterogeneous sets of 8 micron paramagnetic Beads. The beads are infused with various ratios of fluorescent dyes creating unique bead sets. Beads are coated with recombinant and /or native antibodies, antigens specific to a certain assay. Bead sets are then mixed together into a single reagent pack for testing and the simultaneous detection of multiple analytes from a single sample The system uses a dual laser detection system to process a minimum 150 assay readings per analyte. Detection system is capable of reading 100,000 beads per minute.
Notes:
xMap technology also used on Luminex MAGPIX device.
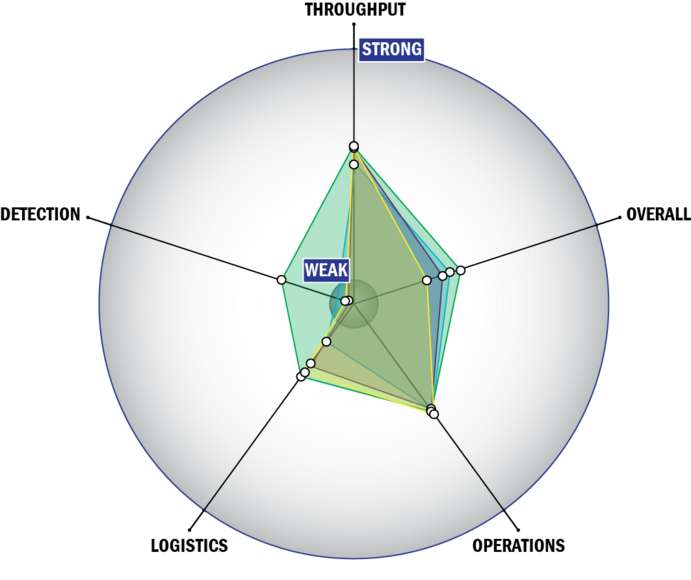
Evaluation Criteria
THROUGHPUT:
- Between 30 and 60 minutes for detection
- Multiple samples, multiple tests/sample per run
- 349-96 samples every 2 hours
- The system or device is currently fully automated
- Device or system is intended for multiple detection assays
- 5 or more solutions, buffer, eluents, and/or reagents
- 2 components
- 5-10 minutes is required for set-up
- 1-2 steps are required for detection
LOGISTICS:
- More than a day of training and significant technical skills are required
- Larger than a home dishwasher
- More than 50 kg
- Wired connections are available
- System or device has 110V electrical requirement
OPERATIONS:
- Can be used from 4°C to 37°C
- Components must be stored at room temperature (27°C)
- Device must be used in a temperature stable, dry environment for optimum performance
- Between 1 to 3 years shelf life
- 5-10 years expected life
- Results cannot be viewed in real-time
- The system is not capable of autonomy
- The system software is closed and not available for modification
- The system hardware is closed and not available for modification
DETECTION:
- System currently has 510k clearance
- System currently has FDA approval
- Less than 250 µL
- Fair specificity. System has a consistent level of false alarms (5-10%)




 Top Tier
Top Tier Second Tier
Second Tier Third Tier
Third Tier Fourth Tier
Fourth Tier Bottom Tier
Bottom Tier