General Description
Not provided.
Technical Description
The CFX Real-Time PCR detection systems are designed for real-time PCR amplification and detection of DNA or cDNA using fluorescence detection to determine target sample presence, absence or quantity. The systems combine a C1000 thermal cycler with interchangeable 96 well or 384 well modules for singleplex and multiplex detection of fluorophores. Purchase of this instrument conveys a limited non-transferable immunity from suit for the purchaser's own internal research and development and for use in human in vitro diagnostics and all other applied fields except veterinary diagnostics. The CFX Automation System enables the automated loading of multiple reaction plates into the CFX system allowing fully automated running sample amplification and detection
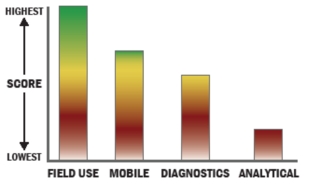
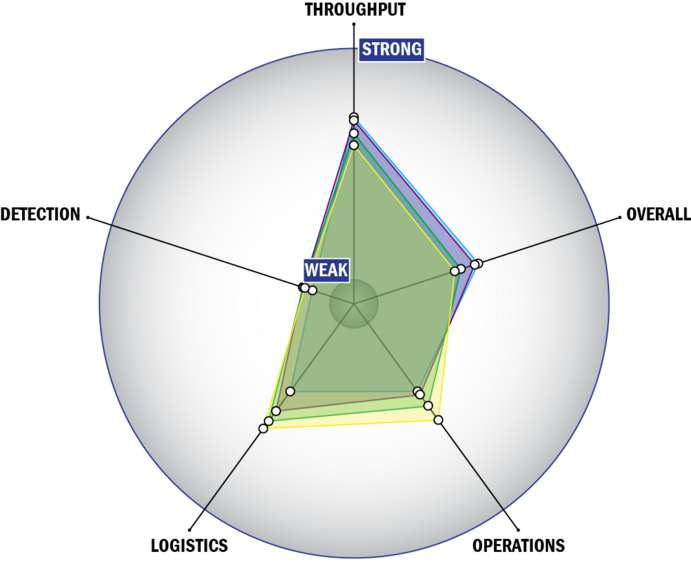
Evaluation Criteria
THROUGHPUT:
- Between 30 and 60 minutes for detection
- Multiple samples, multiple tests/sample per run
- 349-96 samples every 2 hours
- The system could easily be adapted into a fully automated system
- Device or system is intended for multiple detection assays
- 2 solutions, buffer, eluents, and/or reagents
- 2 components
- Less than 5 minutes is required for set-up
- 3-5 steps are required for detection
LOGISTICS:
- An afternoon of training and some technical skills required
- Approximately the size of a toaster
- Between 25 and 50 kg
- Wired connections are available
- System or device has 110V electrical requirement
- Battery life
OPERATIONS:
- Can be used from 25°C to 37°C
- Components must be frozen (-20°C)
- Device or system has peak performance at normal relative humidity conditions
- Results can be viewed in real-time
- The system or device is currently fully autonomous
- The system software is closed and not available for modification
- The system hardware is closed and not available for modification
DETECTION:
- Less than 10 µL
- Manual kit not integrated with the system handles spore lysis




 Top Tier
Top Tier Second Tier
Second Tier Third Tier
Third Tier Fourth Tier
Fourth Tier Bottom Tier
Bottom Tier